NYU Langone Health’s Groundbreaking Transplant Offers Hope amid Organ Shortages
On Oct. 19, 2021, the first investigational transplant of a genetically engineered, nonhuman kidney to a human body…
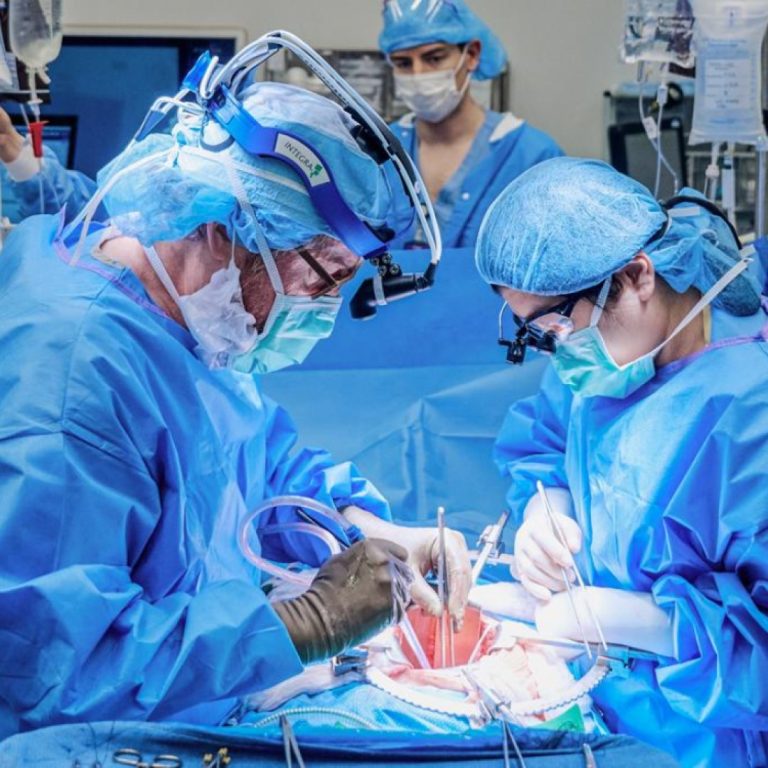
On Oct. 19, 2021, the first investigational transplant of a genetically engineered, nonhuman kidney to a human body…

On Oct. 18, 2021, Gilead Sciences announced that the Food and Drug Administration had approved a new low-dose…

On Oct. 18, 2021, Athenex and the Center for Cell and Gene Therapy at Baylor College of Medicine,…

On Oct. 18, 2021, Pfizer and BioNTech announced that the European Medicines Agency’s Committee for Human Medicinal Products…

On Oct. 15, 2021, Roche announced that the U.S. Food and Drug Administration (FDA) had approved Tecentriq (atezolizumab)…

On Oct. 15, 2021, Pfizer and BioNTech announced they had submitted data supporting the vaccination of children 5…

On Oct. 13, 2021, the World Health Organization (WHO) honoured the late Henrietta Lacks with a WHO Director-General’s…

On Oct. 12, 2021, National Resilience announced a strategic collaboration with Children’s Hospital of Philadelphia (CHOP) to implement…

On Oct. 11, 2021, scientists at Washington University School of Medicine in St. Louis announced they had developed…
On Oct. 7, 2021, PerkinElmer announced that the U.S. Food and Drug Administration (FDA) had issued Emergency Use…

On Oct. 4, 2021, the Fred & Pamela Buffett Cancer Center announced it had successfully renewed its National…

On Oct. 4, 2021, Pfizer and BioNTech announced that the Committee for Medicinal Products for Human Use (CHMP)…

On Oct. 1, 2021, Merck and Ridgeback Biotherapeutics announced that molnupiravir (MK-4482, EIDD-2801), an investigational oral antiviral medicine,…
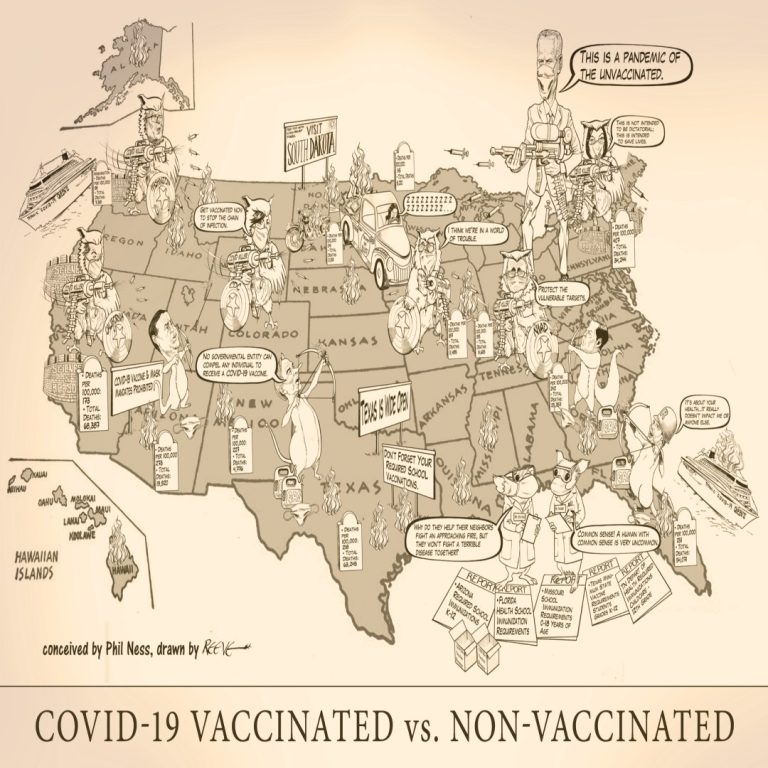
This cartoon illustrates the various issues facing individuals across the U.S. who are vaccinated against COVID-19 verus those…

On Sept. 29, 2021, Pfizer and BioNTech announced they had submitted data to the U.S. Food and Drug…
On Sept. 29, 2021, Regeneron announced that the New England Journal of Medicine (NEJM) published positive detailed results…

On Sept. 29, 2021, Roche confirmed positive data from the phase II/III 2066 study, investigating Ronapreveル (casirivimab and…

On Sept. 29, 2021, the University of Pennsylvania announced that the Breakthrough Prize in Life Sciences was awarded…

On Sept. 28, 2021, Soligenix announced publication of pre-clinical immunogenicity studies for CiVax (heat stable COVID-19 vaccine program)…

On Sept. 23, 2021, Tonix Pharmaceuticals announced it had expanded its research collaboration with Columbia University. The research…
On Sept. 22, 2021, Gilead Sciences announced positive results from a Phase 3 randomized, double-blind, placebo-controlled trial to…

On Sept. 22, 2021, Pfizer and BioNTech announced plans to expand their agreement with the U.S. government by…

On Sept. 17, 2021, Zoo Atlanta reported that of the 20 gorillas at the Zoo, 18 showed clinical…

On Sept. 15, 2021, the National Institutes of Health (NIH) awarded nearly $470 million to build a national…

On Sept. 14, 2021, Regeneron announced an agreement with the U.S. Department of Health and Human Services and…

On Sept. 13, 2021, Twist Bioscience announced and Adicet Bio announced a collaboration to accelerate the discovery of…

On Sept. 9, 2021, the National Institutes of Health (NIH) announced that it was providing approximately $185 million…

On Sept. 8, 2021, National Resilience announced an agreement to manufacture mRNA for the Moderna COVID-19 vaccine. Under…

On Sept. 7, 2021, Takeda Pharmaceutical announced the Government of Japan’s Ministry of Health, Labour and Welfare purchase…

On Sept. 6, 2021, Pfizer and BioNTech announced that they had submitted a variation to the European Medicines…