Pfizer received U.S. FDA Emergency Use Authorization for novel COVID-19 oral antiviral treatment
On Dec. 22, 2021, Pfizer announced that the U.S. Food and Drug Administration (FDA) had authorized the emergency…

On Dec. 22, 2021, Pfizer announced that the U.S. Food and Drug Administration (FDA) had authorized the emergency…
On Dec. 21, 2021, the University of Oxford’s vaccine manufacturing research team published a pre-print paper demonstrating the…

On Dec. 20, 2021, Pfizer and BioNTech announced an agreement had been reached with the European Commission (EC)…

On Dec. 14, 2021, Pfizer announced final results from an analysis of all 2,246 adults enrolled in its…

On Dec. 9, 2021, CytoDyn announced that it had submitted a Phase 3, randomized, double blind, placebo controlled…

On Dec. 8, 2021, Pfizer and BioNTech announced results from an initial laboratory study demonstrating that serum antibodies…

On Dec. 8, 2021, in a large-scale study of people from diverse ancestries, researchers narrowed down the number…

On Dec. 7, 2021, Rockefeller University scientists announced a study had demonstrated the therapeutic potential of an unusual…

On Dec. 5, 2021, Sorrento Therapeutics announced the peer-reviewed publication of a series of novel SARS-CoV-2 MPro inhibitors…

On Dec. 3, 2021, a report from the U.S. Department of Health and Human Services (HHS) found that…
On Dec. 1, 2021, T2 Biosystems announced that its T2SARS-CoV-2 Panel detected the Omicron COVID-19 variant (B.1.1.529). The…
On Dec. 1, 2021, SIGA Technologies announced that Health Canada had approved oral TPOXX (tecovirimat) as an extraordinary…
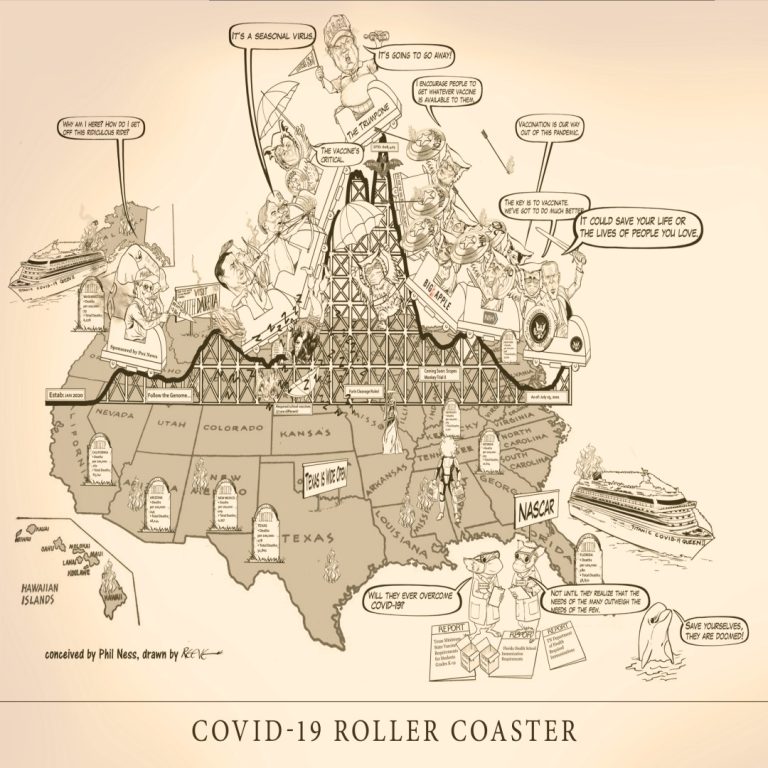
The COVID-19 Roller Coaster is a wild ride, strap yourself in and hold on… Cast of Characters: Senator…

On Nov. 26, 2021, Merck provided an update on the MOVe-OUT study of molnupiravir, an investigational oral antiviral…
On Nov. 18, 2021, Pfizer announced an agreement with the U.S. government to supply 10 million treatment courses…

On Nov. 17, 2021, Pacific Northwest National Laboratory (PNNL) announced researchers had compiled the most comprehensive road map…

On Nov. 17, 2021, GlaxoSmithKline and Vir Biotechnology announced U.S. government contracts totalling approximately $1 billion (USD) to…

On Nov. 16, 2021, Pfizer announced it had submitted an Emergency Use Authorization (EUA) of its investigational oral…

On Nov. 16, 2021, Illumina and Genetic Alliance announced the creation of the iHope Genetic Health program aimed…

On Nov. 14, 2021, the Calgary city council voted to reinstate fluoridation in Calgary’s waters. The count was…

On Nov. 8, 2021, Regeneron announced that the European Commission had approved the casirivimab and imdevimab antibody cocktail,…

On Nov. 8, 2021, Cocrystal Pharma announced that its SARS-CoV-2 main protease inhibitors showed potent in vitro pan-viral…

On Nov. 5, 2021, the USDA’s (USDA) National Veterinary Services Laboratories announced confirmation of SARS-CoV-2 in two spotted…

On Nov. 5, 2021, Pfizer announced it was investigational novel COVID-19 oral antiviral candidate, PAXLOVID, significantly reduced hospitalization…

On Nov. 5, 2021, Chugai Pharmaceutical, announced that it had obtained approval from the Ministry of Health, Labour…

On Oct. 29, 2021, Oregon Health & Science University’s (OHSU) board of directors approved a project to expand…

On Oct. 28, 2021, Pfizer and BioNTech announced that the U.S. government had purchased 50 million more doses…

On Oct. 25, 2021, Moderna announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human…

On Oct. 21, 2021, Pfizer and BioNTech announced topline results from a Phase 3 randomized, controlled trial evaluating…

On Oct. 20, 2021, Quest Diagnostics announced it had formed an agreement with the Texas Department of State…