WHO launches first-ever guidelines on meningitis diagnosis, treatment and care
On Apr. 10, 2025, the World Health Organization (WHO) published its first-ever global guidelines for meningitis diagnosis, treatment…
On Apr. 10, 2025, the World Health Organization (WHO) published its first-ever global guidelines for meningitis diagnosis, treatment…
On Apr. 10, 2025, the U.S. Food and Drug Administration announced it is taking a groundbreaking step to…

On Apr. 10, 2025, Novartis, a leading global innovative medicines company, announced a planned $23 billion investment over…
On Apr. 9, 2025, a team of neuro scientists scientists published findings from the Machine Intelligence from Cortical…

On Apr. 9, 2025, Roswell Park Comprehensive Cancer Center achieved textbook outcomes for 90% of 150 consecutive robot-assisted,…

On Apr. 8, 2025, the National Security Commission on Emerging Biotechnology delivered its major report and action plan…

On Apr. 8, 2025, the Hawaiʻi Department of Health (DOH) State Laboratories Division confirmed a case of measles…

On Apr. 7, 2025, in a landmark study, researchers at University of Nebraska Medical Center (UNMC) announced they…
On Apr. 4, 2025, a team of researchers led the University of Utah Health reported that by tracking…

On Apr. 4, 2025, the World Health Organization (WHO) announced that over the past two days, it convened…

On Apr. 3, 2025, a team led by researchers at Baylor College of Medicine and the Jan and…

On Apr. 3, 2025, a Stanford-led study has found that the gene CXCL12 is connected to the posterior…

On Apr. 3, 2025, The International Journal of Neonatal Screening, an international, peer-reviewed journal focused on newborn screening…

On Apr. 3, 2025, a collaborative research led by investigators at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center…

On Apr. 2, 2025, Northwestern University engineers announced they have developed a pacemaker so tiny that it can…

On Apr. 1, 2025, layoff notices began arriving for thousands of employees of the sprawling U.S. Department of…

On Mar. 31, 2025, a team of researchers from UC Berkeley and UC San Francisco announced they have…

On Mar. 27, 2025, The Hospital for Sick Children (SickKids) announced it is harnessing the transformative potential of…
On Mar. 27, 2025, the California Institute for Regenerative Medicine (CIRM), one of the world’s largest institutions dedicated…

On Mar. 27, 2025, a Nature survey of more than 1,200 scientists, three-quarters of the total respondents are…

On Mar. 26, 2025, researchers announced they have discovered a new antibiotic molecule that targets a broad range…

On Mar. 26, 2025, the Biotechnology Innovation Organization (BIO) released results from a membership survey that underscores the…

On Mar. 25, 2025, Merck announced it has signed a licensing agreement for a heart disease drug with…

On Mar. 25, 2025, GSK announced that the US Food and Drug Administration (FDA) has approved Blujepa (gepotidacin)…

On Mar. 24, 2025, LifeScienceHistory.com published the cartoon “Milestones in Fluoride” illustrating the facts behind the naturally occurring…
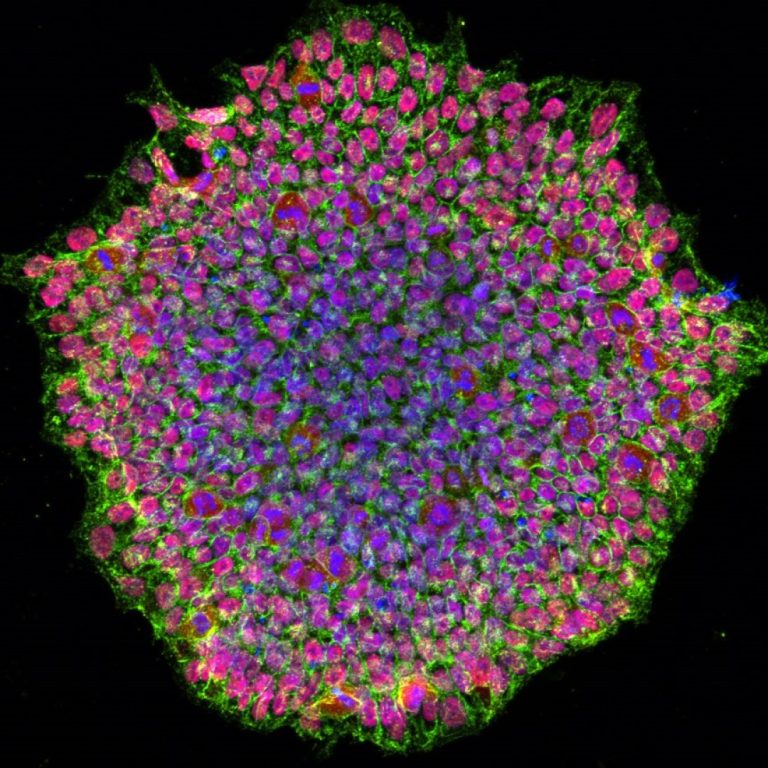
On Mar. 24, 2025, Hideyuki Okano, a stem-cell scientist at Keio University in Tokyo, and his colleagues announced…

On Mar. 24, 2025, scientists at the UCLA Health Jonsson Comprehensive Cancer Center and the University of Toronto…

On Mar. 20, 2025, Rice University, electrical and computer engineer Kaiyuan Yang unveiled a first-of-its-kind authentication protocol for…
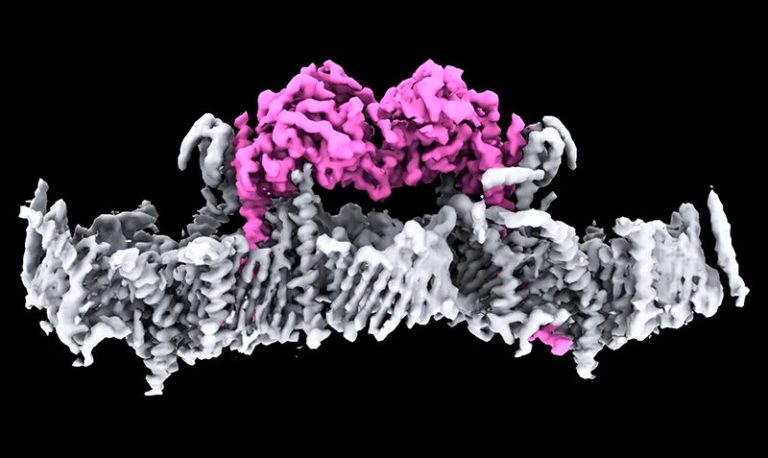
On Mar. 14, 2025, researchers from the Walter and Eliza Hall Institute of Medical Research (WEHI) announced they…

Mar. 13, 2025, Mallinckrodt and Endo,announced that they had entered into a definitive agreement to combine in a stock…