U.S. healthcare spending soars to over $5 trillion in 2024
On Jan. 14, 2026, U.S. healthcare spending rose by 7.2% to $5.3 trillion in 2024 from $4.9 trillion…

On Jan. 14, 2026, U.S. healthcare spending rose by 7.2% to $5.3 trillion in 2024 from $4.9 trillion…
On Nov. 19, 2025, Precise Bio announced the first patient has been successfully treated with PB-001, the Company’s…

On Oct. 24, 2025, Eli Lilly and Adverum Biotechnologies announced a definitive agreement for Lilly to acquire Adverum,…

On Oct. 20, 2025, Glaukos announced today the U.S. Food and Drug Administration (FDA) approved its Epioxa™ HD…

On Sept. 25, 2025, Glaukos, an ophthalmic pharmaceutical and medical technology company focused on novel therapies for the…
On Sept. 3, 2025, The International Brain Laboratory (IBL) researchers published their findings today in two papers in Nature, revealing…

On Aug. 21, 2025, the Institute for Health Metrics and Evaluation (IHME) announced a recent study aimed to…

On May 29, 2025, The California Institute for Regenerative Medicine (CIRM), one of the world’s largest institutions dedicated…

On My 22, 2025, team led by scientists at the University of Science and Technology of China (USTC)…
On May 6, 2025, in a first-of-its-kind surgery, a multidisciplinary team led by a University of Maryland Medical…
On Mar. 6, 2025, the U.S. Food and Drug Administration (FDA) announced the approval of ENCELTO (revakinagenetaroretcel-lwey), a…

On Feb. 19, 2025, Alcon announced the full U.S. commercial availability of Voyager™ DSLT, the first and only…
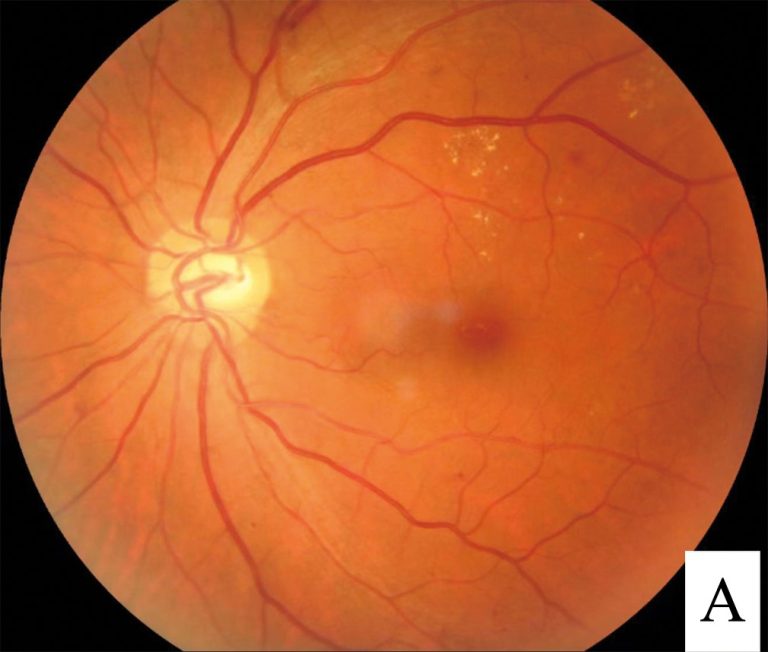
On Feb. 4, 2025, Genentech announced that the U.S. Food and Drug Administration (FDA) had approved Susvimo® (ranibizumab…

On Dec. 2, 2024, the University of Colorado Anschutz Medical Campus announced it will receive up to $46…

On Oct. 18, 2024, a study published in JAMA Ophthalmology, found that approximately 4.22 million people in the…

On Sep. 10, 2024, researchers at the Karolinska Institute in Sweden announced a study that shows that gene…

Black History Month Celebrates the pioneering accomplishments of James McCune Smith, Rebecca Lee Crumpler, Alexa Canady and Patricia…

On Apr. 26, 2024, the California Institute for Regenerative Medicine (CIRM) announced it had approved awarding nearly $31…
On Aug. 25, 2023, Harvardメs Center for Brain Science announced the ceation of an eye atlas that pinpoints…

On Jun. 2, 2022, the National Institutes of Health (NIH) reported that the Age-Related Eye Disease Studies (AREDS…
On Sept. 18, 2019, researchers from the University of California, Los Angeles (UCLA) announced developing a device geared…

On Jun. 7, 2018, the Wold Foundation of Wyoming announced a gift of $2.5 million to help in…

On Apr. 24, 2015, researchers led by David Huang, M.D., Ph.D. at Oregon Health Sciences University’s (OHSU) Casey…

On Aug. 10. 2012, Genentech announced that Lucentis (ranibizumab injection) was approved by the U.S. Food and Drug…

In 2006, the Board of Trustees of the Northwest Lions Foundation for Sight & Hearing renamed Northwest Lions…

On Jan. 29, 2004, researchers at Oregon Health & Science University (OHSU) School of Medicine’s Casey Eye Institute…

On Oct. 21, 2003, scientists at Oregon Health Sciences University’s (OHSU) Casey Eye Institute announced they had found…

In 2001, the Northwest Lions Eye Bank merged with the Montana Eye Bank. SightLife, based in Washington state,…

In 1997, the University of Iowa established the Center for Macular Degeneration. The Center was later renamed the…

In 1996, Dr. Brenda Gallie identified the cause of drug-resistant childhood cancer of the retina, leading to an…