Menopause linked to loss of grey matter in the brain, poorer mental health and sleep disturbance
On Jan. 27, 2026, a University of Cambridge study shows menopause is linked to reductions in grey matter…
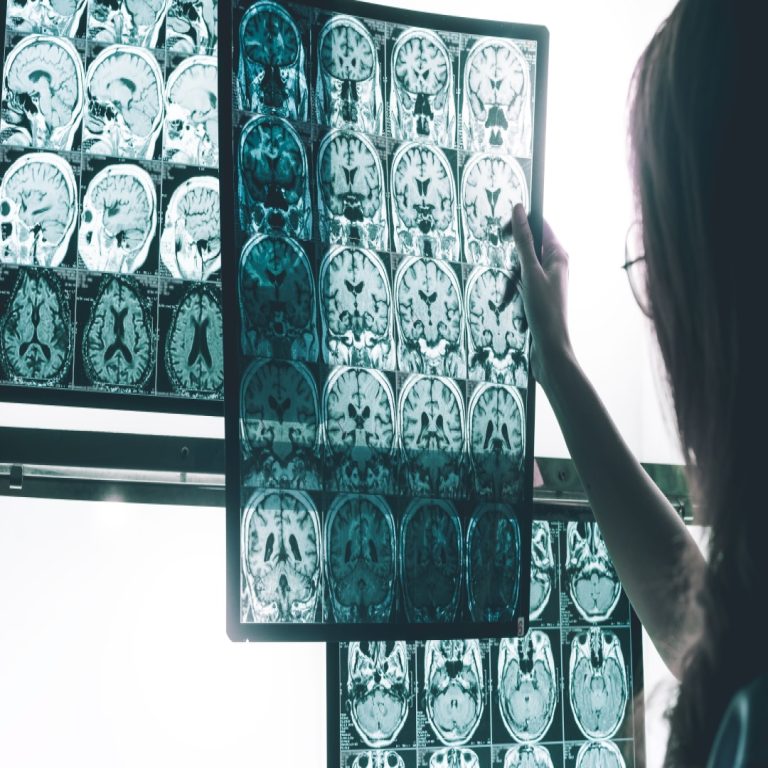
On Jan. 27, 2026, a University of Cambridge study shows menopause is linked to reductions in grey matter…
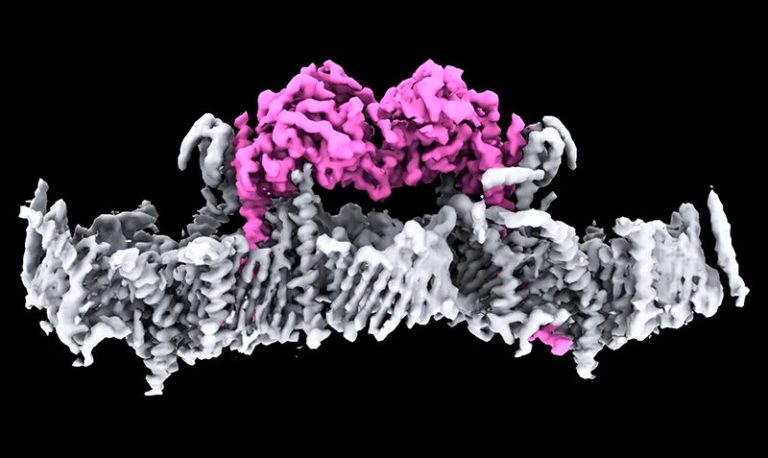
On Jan. 22, 2026, researchers backed by a Knight Initiative for Brain Resilience Catalyst Momentum Award have laid out…
On Jan. 21, 2026, Rice University launched the Global Brain Economy Initiative (GBEI) during the annual meeting of…
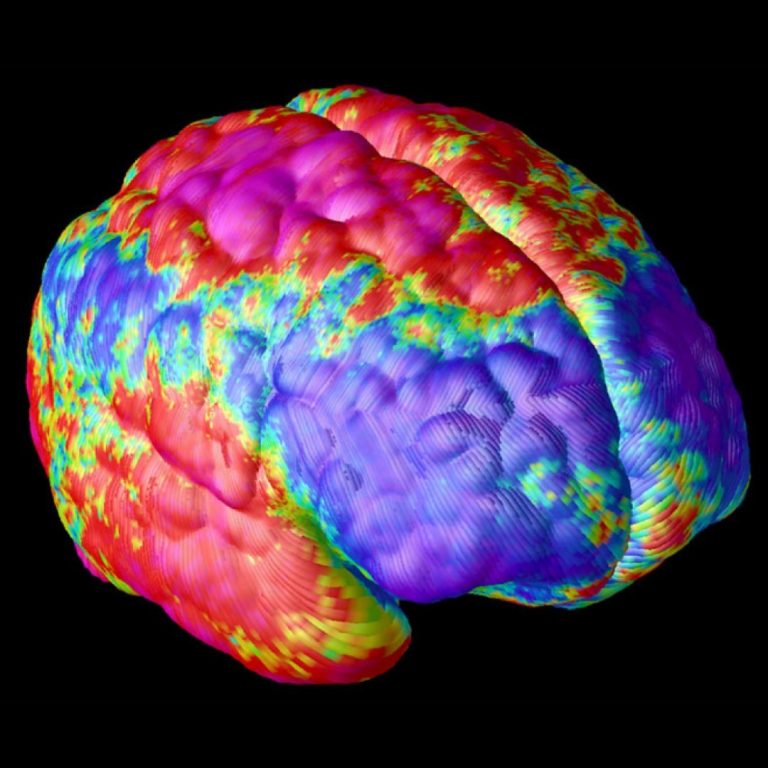
On Jan. 20, 2026, researchers announced they have identified two biomarkers — verbal learning and identifying other peoples’…
On Jan. 14, 2026, a new study published in JAMA Network suggest that health care costs associated with…

On Jan. 7, 2025, Eli Lilly and Ventyx Biosciences, a San Diego-based clinical-stage biopharmaceutical company focused on developing…
On Dec. 17, 2025, A survey of Alzheimer’s disease prevalence in Norway confirms earlier estimates and might show…

On Dec. 12, 2025, a Japanese research team announced it has successfully reproduced the human neural circuit in…

On Dec. 11, 2025, a new analysis from a World Health Organization(WHO) global expert committee on vaccine safety…

On Dec. 9, 2025, officials from the Canada Economic Development for Quebec Regions, and the Minister of Industry,…

On Dec. 5, 2025, an annual report published by the Institutes of Science and Development of the Chinese…

On Dec. 5, 2025, Amneal Pharmaceuticals announced new positive interim results from its ongoing Phase 4 ELEVATE-PD study….

On Nov. 25, 2025, Governor Kathy Hochul announced the University at Buffalo (UB) will receive $50 million from…

On Nov. 24, 2025, a study published in the New England Journal of Medicine show that a test…

On Nov. 21, 2025, the World Health Organization (WHO) reported that from 1 January to 2 November 2025,…

On Nov. 21, 2025, state health officials from California, Oregon, Hawaii and Washington issued a statement in response…

On Nov. 20, 2025, the National Institutes of Health (NIH) has canceled funding for at least 383 clinical…

On Nov. 19, 2025, a study by researchers from Nanyang Technological University (NTU) Singapore announced they had discovered…

On Nov. 19, 2025, Novartis, a leading global innovative medicines company, announced plans to expand its operations in…

On Nov. 17, 2025, a JAMA published study found that more than 74,000 people have had their lives…
On Nov. 12, 2025, Allen Institute researchers and engineers have unlocked an the world’s most comprehensive AI-powered tool…

On Nov. 11, 2025, China introduced a visa that will allow young foreign researchers in science, technology, engineering…
On Nov. 10, 2025, a study led by the University of Liverpool reported that existing evidence does not…
On Nov. 7, 2025, Fang Lei, Ph.D., a long-time professor with Texas A&M University’s chemistry department, has returned…
On Nov. 5, 2025, researchers announced that reading a person’s mind using a recording of their brain activity…

On Nov. 5, 2025, MIT researchers announced they have developed a new 3D human brain tissue platform, the…

On Nov. 3, 2025, Cornell researchers and collaborators have developed a neural implant so small that it can…

On Oct. 30, 2025, the California Institute for Regenerative Medicine (CIRM) approved awarding $27 million through three grants…
On Oct. 29, 2025, the CWTS Leiden ranking show that Harvard University dropped to third place in the…

On Oct. 29, 2025, Eli Lilly announced a planned investment of more than $1.2 billion to expand and…