Super microscope showed nanoscale biological process for the first time
On Nov. 12, 2024, researchers led by Nico Sommerdijk from Radboud university medical center in Nijmegen (Netherlands) announced…
On Nov. 12, 2024, researchers led by Nico Sommerdijk from Radboud university medical center in Nijmegen (Netherlands) announced…
On Oct. 30, 2024, an international research team led by the University of Bonn reported results of a…
On Oct. 11, 2024, scientists at the Korea Advanced Institute of Science and Technology (KAIST) announced they had…

On Oct. 1, 2024, the National Academy of Medicine (NAM) announced a study showing that although the U.S….
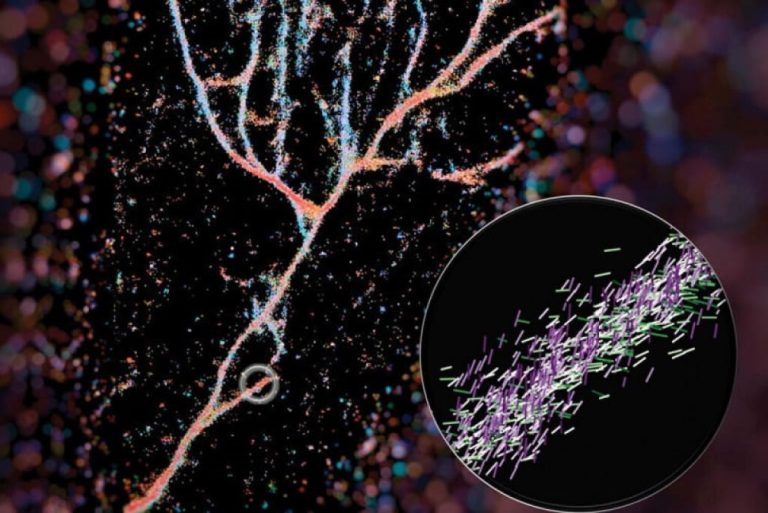
On Apr. 26, 2024, engineers at Washington University in St. Louis announced a new imaging technique that give…

On Dec. 14, 2023, the Department of Energy’s (DOE) Pacific Northwest National Laboratory (PNNL) announced that it had…

On Sept. 15, 2023, researchers at the National Institute of Allergy and Infectious Diseases (NIAID) announced they had…

On Aug. 22, 2023, Alberta Innovates announced that more than $13.6 million will go to 19 partners, across…

On Mar. 28, 2023, the American Chemical Society awarded The Priestley Medal to Cato T. Laurencin “to recognize distinguished…

On Feb. 10, 2023, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for…
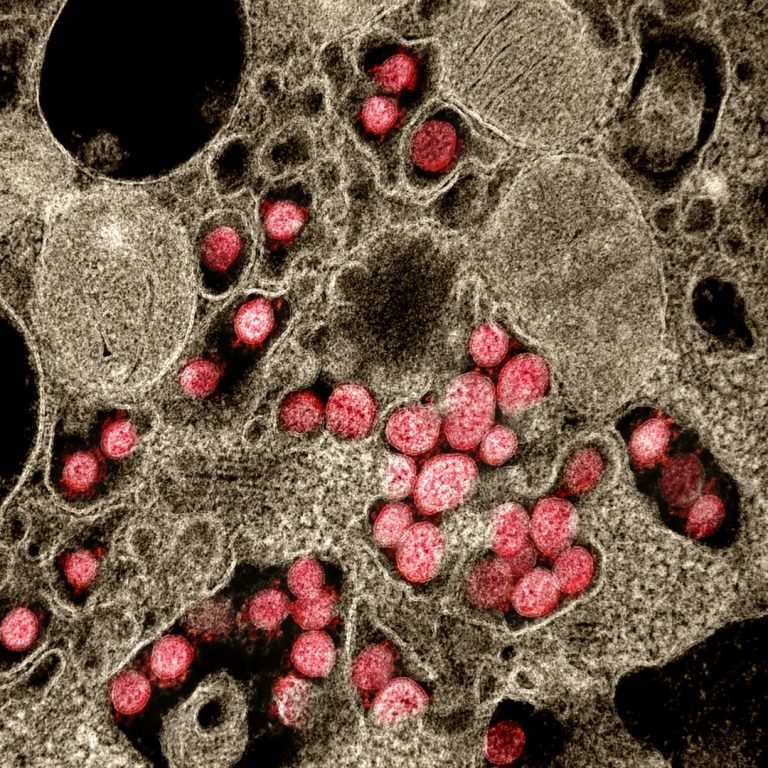
On Oct. 25, 2022, Pacific Northwest National Laboratory (PNNL) announced that scientists have shown that they can detect…

On Feb. 10, 2022, Novavax announced that NVX-CoV2373, its recombinant nanoparticle protein-based COVID-19 vaccine, achieved its primary effectiveness…

On Jan. 28, 2022, Novavax and Israel’s Ministry of Health announced an agreement for the purchase of NVX-CoV2373,…
On Jan. 10, 2022, Novavax and Serum Institute of India announced a regulatory submission to the South African…

On Jan. 4, 2022, Tonix Pharmaceuticals announced an exclusive option agreement and research collaboration with Kansas State University…

On Dec. 28, 2021, Novavax and Serum Institute of India announced that the Drugs Controller General of India…

On Dec. 23, 2021, Novavax and SK bioscience announced the expansion of the companies’ collaboration and license agreements…

On Dec. 21, 2021, Novavax announced that the first booster doses of NVX-CoV2373, the company’s recombinant nanoparticle protein-based…

On Dec. 20, 2021, Novavax announced that the World Health Organization (WHO) had granted a second Emergency Use…

On Dec. 17, 2021, Novavax and SK bioscience announced that the World Health Organization (WHO) had granted Emergency…

On Dec. 15, 2021, Novavax announced the submission of a New Drug Application to the Ministry of Health,…

On Dec. 7, 2021, Rockefeller University scientists announced a study had demonstrated the therapeutic potential of an unusual…

On Nov. 17, 2021, Novavax and and Serum Institute of India announced that the Philippine Food and Drug…

On Nov. 17, 2021, Zosano Pharma announced that the Philippine Food and Drug Administration had granted emergency use…

On Nov. 15, 2021, Novavax and SK bioscience announced submission of a Biologics License Application (BLA) for Novavax’…

On Sept. 21, 2021, the Medicines Patent Pool (MPP) announced that it had signed a licence agreement with…

On Nov. 4, 2021, Novavax announced the completion of its rolling submission to the World Health Organization (WHO)…

On Nov. 1, 2021, Novavax and Serum Institute of India announced that the National Agency of Drug and…

On Sept. 23, 2021, Novavax with its partner, Serum Institute of India announced a regulatory submission to the…

On Sept. 21, 2021, the Medicines Patent Pool (MPP) announced that it had signed a licence agreement with…