Queensland University leads international team researching ancient Australian plant to help in production of COVID-19 vaccine
On Apr. 7, 2020, the Queensland University of Technology (QUT) announced that it is leading an international team…

On Apr. 7, 2020, the Queensland University of Technology (QUT) announced that it is leading an international team…

On Apr. 7, 2020, researchers at the University of Iowa and the University of Georgia announced they had…

On Apr. 7, 2020, Harvard University, Massachusetts Institute of Technology, and Stanford University announced they had established and…

On Apr. 6, 2020, Intravacc announced development of an intranasal vaccine against COVID-19 that will be developed through…

On Apr. 6, 2020, 3M announced a partnership with Cummins to increase the production of high efficiency particulate…

On Apr. 6, 2020, BioReference Laboratories, an OPKO Health company, announced that it will continue to prioritize COVID-19…

On Apr. 6, 2020, Biotest, BPL Group, LFB, and Octapharma joined an alliance formed by CSL Behring and…

On Apr. 6, 2020, the USDA confirmed SARS-CoV-2 (the virus that causes COVID-19 in humans) in one tiger…

On Apr. 6, 2020, Mateon Therapeutics announced an update on its rapid antiviral response program targeting coronaviruses, initially…

On Apr. 6, 2020, OncoSec Medical announced that Providence Cancer Institute, a part of Providence St. Joseph Health…

On Apr. 6, 2020, new COVID-19 estimated find that, among European nations, the peak daily death rate from…

On Apr. 6, 2020, AIM ImmunoTech announced a Material Transfer and Research Agreement (MTA) with Shenzhen Smoore Technology…

On Apr. 6, 2020, Innovation Pharma announced that it is engaged in discussions with health care provider networks…

On Apr. 6, 2020, Sema4 announced that it is now performing rapid molecular testing for the detection of…

On Apr. 6, 2020, Getinge announced it was ramping up the production capacity stepwise at its production facility…

On Apr. 6, 2020, GSK and Vir Biotechnology announced a binding agreement to enter into a collaboration to…

On Apr. 6, 2020, IGEA Pharma announced that it was starting to supply of a COVID-19 antibody test…

On Apr. 6, 2020, to address the need for N95 masks, an interdisciplinary research team at the University…

On Apr. 3, 2020, 3M announced that the Administration formally invoked the Defense Production Act to require 3M…

On Apr. 3, 2020, the University of Minnesota announced it had created the stateメs fourth on-site testing facility…

On Apr. 3, 2020, UNMC aunched a groundbreaking mobile app for Apple Iphone to screen large groups of…

On Apr. 3, 2020, Montefiore Health System and Albert Einstein College of Medicine started treating very sick COVID-19…

On Apr. 3, 2020, XBiotech and BioBridge Global announced a collaboration to participate in a FDA investigational program…

On Apr. 2, 2020, a new CDC report, published in the New England Journal of Medicine, showed Clostridioides…
On Apr. 2, 2020, BioAegis Therapeutics announced that it was preparing to evaluate plasma gelsolin replacement in severe…

On Apr. 2, 2020, the FDAラin collaboration with the CDC, the Biodefense and Emerging Infections Research Resources Repository…

On Apr. 2, 2020, Roswell Park Comprehensive Cancer Center and the University at Buffalo (UB) launched a collaborative…

On Apr. 2, 2020, Cerus announced that the University Hospital Basel transfused the first two COVID-19 patients with…
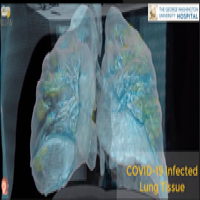
On Apr. 2, 2020, George Washington University Hospital announed it had used Virtual Reality technology to see into…

On Apr. 2, 2020, Amgen and Adaptive Biotechnologies announced a collaboration aimed at helping address the COVID-19 pandemic….