Penn engineers turn toxic fungus into anti-cancer drug
On Jun. 25, 2025, researchers at the University of Pennylvanis’s School of Engineering and Applied Science announced they…

On Jun. 25, 2025, researchers at the University of Pennylvanis’s School of Engineering and Applied Science announced they…

On Jun. 25, 2025, the National Institutes of Health (NIH) moved to reinstate about 900 grants that a…

On Jun. 25, 2025, in the midst of a multi-state measles outbreak, a poll by Harvard T.H. Chan…

On Jun. 25, 2025, the U.S. will announced it will no longer contribute funding to Gavi, a global…

On Jun. 24, 2025, the Oregon Health Authority and county public health officials announced they are investigating a…

On Jun. 24, 2025, the Texas Department of State Health Services (DSHS) reported a total of 750 cases…

On Jun. 24, 2025, the Institute for Health Metrics and Evaluation (IHME) announced results from the Global Burden…

On Jun. 24, 2025, the North Carolina Department of Health and Human Services has confirmed a case of…

On Jun. 23, 2025, Texas A&M research shows wearable health tech can detect infections like COVID-19 and flu…

On Jun. 20, 2025, The U.S. Department of Agriculture’s Animal and Plant Health Inspection Service (APHIS) announced ot…
On Jun. 18, 2025, the U.S. Department of Agriculture announced it has launched an $8.5 million sterile New…

On Jun. 18, 2025, researchers at the University of Washington (UW) School of Medicine announced that a highly…

On Jun. 18, 2025, Gilead Sciences announced that the U.S. Food and Drug Administration (FDA) has approved Yeztugo®…

On Jun. 17, 2025, the New Mexico Department of Health (NMDOH) announced that wastewater in Deming has tested…

On Jun. 17, 2025, the World Health Organization (WHO) reported that from January 1, 2025 to May 25,…

On Jun. 17, 2025, the Texas Department of State Health Services (DSHS) reported a total of 750 cases…
On Jun. 13, 2025, the Washington State Department of Health (DOH) reported that the state experienced a dramatic…

On Jun. 12, 2025, Moderna announced that the U.S. Food and Drug Administration (FDA) has approved mRESVIA® (mRNA-1345),…

On Jun. 12, 2025, U.S. Health Secretary Robert Kennedy Jr. named eight members to serve on a key…

On Jun. 11, 2025, the Montana Department of Public Health and Human Services (DPHHS) reported a total of…

On Jun. 11, 2025, The Iowa Department of Health and Human Services (HHS) is reporting the state’s third…

On Jun. 11, 2025, Novavax announced results of the initial cohort of its COVID-19-Influenza Combination (CIC) and stand-alone trivalent…
On Jun. 10, 2025, the Texas Department of State Health Services (DSHS) reported 744 cases of measles have…

On Jun. 9, 2025, Health and Human Services Secretary Robert F. Kennedy Jr. has fired all 17 members…

On Jun. 9, 2025, Oakland County Health Division in Michigan is notifying the public about a measles exposure…
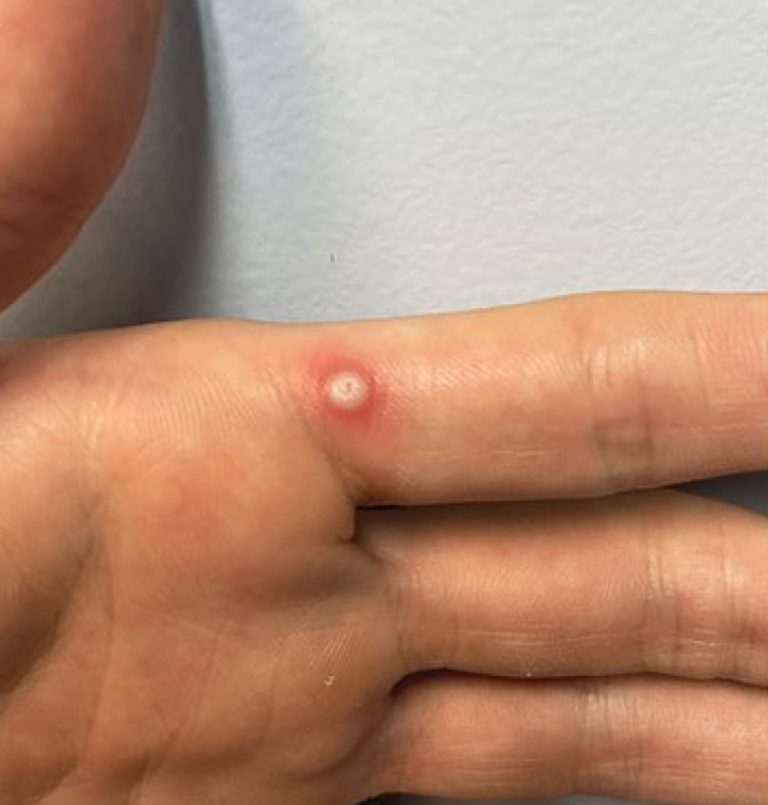
On Jun. 9, 2025, the World Health Organization (WHO) said the mpox outbreak is still a public health…

On Jun. 9, 2025, Merck announced the U.S. Food and Drug Administration (FDA) has approved ENFLONSIA™ (clesrovimab-cfor) for…

On Jun. 9, 2025, the Colorado Department of Public Health and Environment, the Arapahoe County Public Health Department,…

On Jun. 9, 2025, the Navajo County Public Health Services District (NCPHSD), in coordination with the Arizona Department…

On Jun. 6, 2025, the Texas Department of State Health Services (DSHS) reported 742 cases of measles have…