Moderna announced preliminary booster data and updated strategy to address Omicron variant
On Dec. 20, 2021, Moderna announced preliminary neutralizing antibody data against the Omicron variant following the Company’s booster…
On Dec. 20, 2021, Moderna announced preliminary neutralizing antibody data against the Omicron variant following the Company’s booster…

On Dec. 17, 2021, Novavax and SK bioscience announced that the World Health Organization (WHO) had granted Emergency…

On Dec. 15, 2021, Novavax announced the submission of a New Drug Application to the Ministry of Health,…

On Dec. 14, 2021, Pfizer announced final results from an analysis of all 2,246 adults enrolled in its…

On Dec. 13, 2021, Moderna announced an agreement with the Australian Government to build a state-of-the-art messenger RNA…

On Dec. 13, 2021, Novavax announced that it had submitted a regulatory filing to the Ministry of Health…

On Dec. 10, 2021, Moderna announced an amendment to its existing contract with Gavi, the Vaccine Alliance, to…

On Dec. 9, 2021, CytoDyn announced that it had submitted a Phase 3, randomized, double blind, placebo controlled…

On Dec. 9, 2021, Moderna announced the launch of its Artificial Intelligence Academy, an innovative initiative that will…

On Dec. 7, 2021, Rockefeller University scientists announced a study had demonstrated the therapeutic potential of an unusual…

On Dec. 5, 2021, Sorrento Therapeutics announced the peer-reviewed publication of a series of novel SARS-CoV-2 MPro inhibitors…

On Dec. 5, 2021, Roche announced that its planned to launch the SARS-CoV-2 & Flu A/B Rapid Antigen…

On Dec. 3, 2021, a report from the U.S. Department of Health and Human Services (HHS) found that…
On Dec. 1, 2021, T2 Biosystems announced that its T2SARS-CoV-2 Panel detected the Omicron COVID-19 variant (B.1.1.529). The…

On Dec. 1, 2021, Moderna announced a revised supply agreement with the UK government for up to 60…
On Dec. 1, 2021, SIGA Technologies announced that Health Canada had approved oral TPOXX (tecovirimat) as an extraordinary…
On Nov. 30, 2021, Inovio Pharma announced the company was rapidly moving to evaluate its COVID-19 DNA vaccine…

On Nov. 29, 2021, Hologic announced that its three SARS-CoV-2 tests all detect the recently emerged Omicron variant of…

On Nov. 26, 2021, Merck provided an update on the MOVe-OUT study of molnupiravir, an investigational oral antiviral…

On Nov. 25, 2021, Pfizer and BioNTech announced that the Committee for Medicinal Products for Human Use (CHMP)…

On Nov. 24, 2021, Novavax announced its submission to the Singapore Health Sciences Authority for interim authorization of…

On Nov. 24, 2021, Johnson & Johnson announced the U.S. Food and Drug Administration (FDA) had issued Emergency…

On Nov. 22, 2021, the U.S. Food and Drug Administration (FDA) authorized another over-the-counter (OTC) COVID-19 antigen test. The…
On Nov. 22, 2021, Tonix Pharmaceuticals announced the publication of ‘Sangivamycin is highly effective against SARS-CoV-2 in vitro…

On Nov. 19, 2021, the U.S. Food and Drug Administration (FDA) announced that it had extended the emergency…
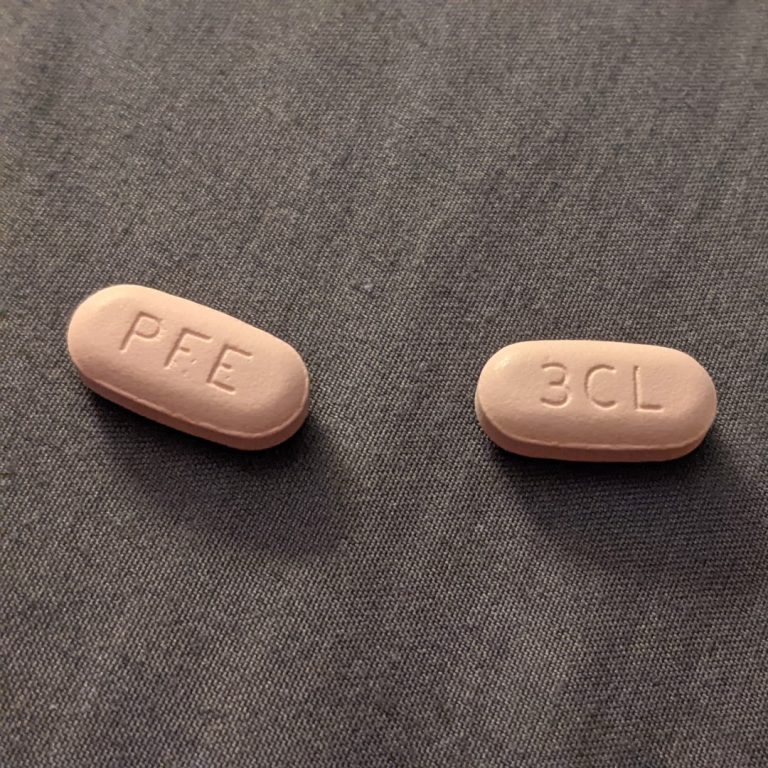
On Nov. 18, 2021, Pfizer announced an agreement with the U.S. government to supply 10 million treatment courses…

On Nov. 17, 2021, Novavax and and Serum Institute of India announced that the Philippine Food and Drug…

On Nov. 17, 2021, Novavax announced that the European Medicines Agency (EMA) had begun its evaluation of an…

On Nov. 17, 2021, Zosano Pharma announced that the Philippine Food and Drug Administration had granted emergency use…

On Nov. 16, 2021, Pfizer announced it had submitted an Emergency Use Authorization (EUA) of its investigational oral…