New York State Department of Health Issued Health Advisory on First Mpox Clade Ib Case Detected in New York
On Feb. 12, 2025, the New York State Department of Health issued a Health Advisory to health care…
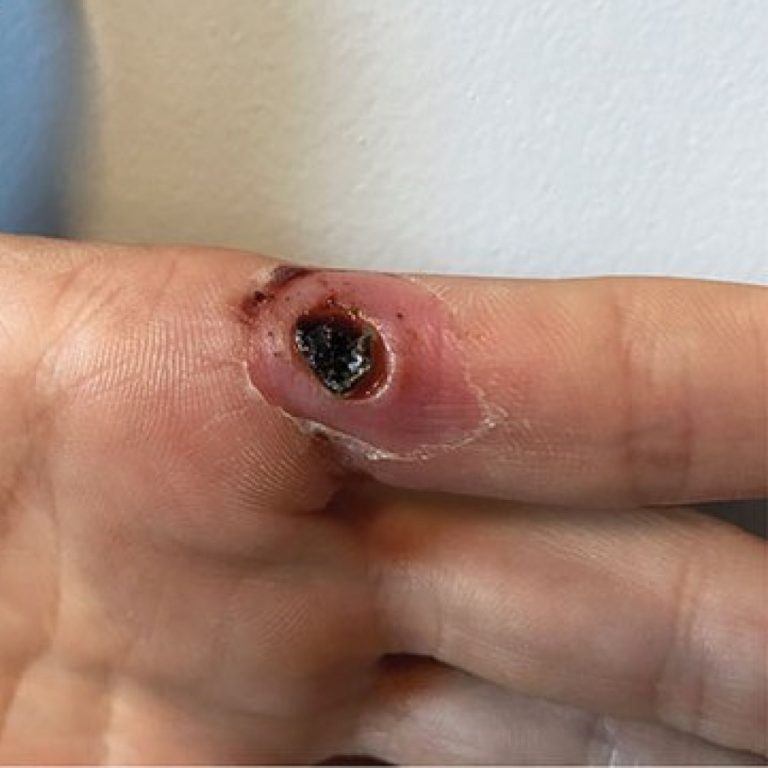
On Feb. 12, 2025, the New York State Department of Health issued a Health Advisory to health care…

On Feb. 11, 2025, the Arizona Department of Agriculture (AZDA) reported working in conjunction with the United States…

On Feb. 8, 2025, the United States Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS)…

On Feb. 7, 2025, an international team of researchers reported Yersinia pestis genome had been recovered from a…

On Feb. 5, 2025, the proposed dismantling of the U.S. Agency for International Development (USAID) could disrupt clinical…
On Feb. 5, 2025, the Spokane Regional Health District announced the first confirmed death from pertussis (whooping cough)…
On Feb. 3, 2025, in a global first, Uganda’s Ministry of Health, the World Health Organization (WHO) and…
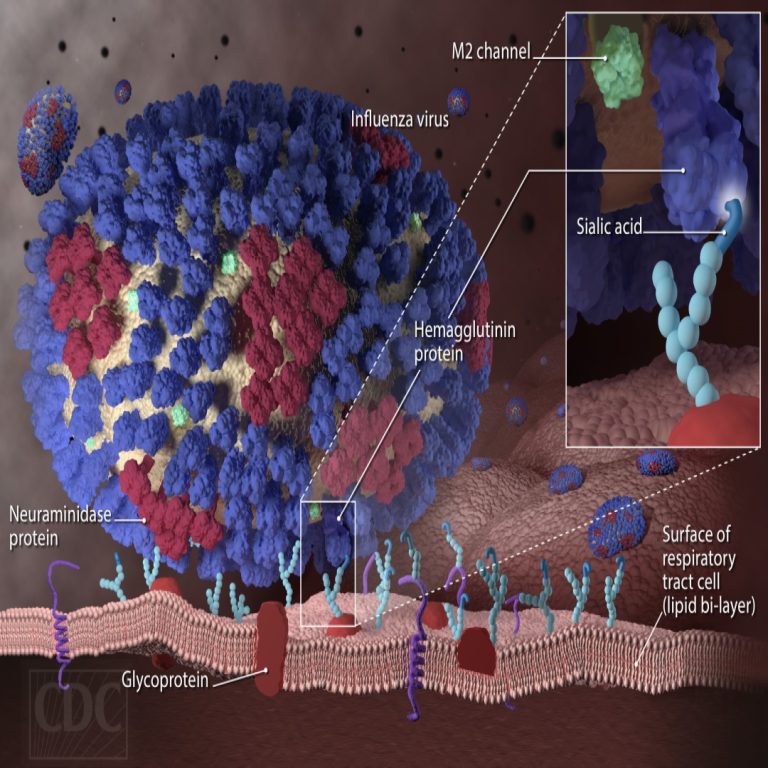
On Jan. 31, 2025, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) confirmed…
On Jan. 31, 2025, the Kansas Department of Health and Environment (KDHE) reported that there were 67 confirmed…

On Jan. 30, 2025, the World Health Organization (WHO) announced it was mobilizing efforts to support national health…

On Jan. 30, 2025, the World Health Organization (WHO) congratulated Niger for having met the criteria for onchocerciasis…
On Jan. 29, 2025, researchers at The University of Queensland announced they have identified the first henipavirus in…

On Jan. 28, 2025, Rose Acre Farms, the nation’s second largest egg producer, reported that tests have confirmed…

On Jan. 27, 2024, the World Organisation for Animal Health (WOAH) reported that highly pathogenic avian influenza (HPAI)…
On Jan. 27, 2025, researchers reported that the H5N1 virus is adapting to new mammalian hosts, raising the…
On Jan. 24, 2025, the World Health Organization (WHO) reported that from January 1 to December, 29, 2024,…

On Jan. 24, 2025, researchers from the Veterans Affairs Portland Health Care System in Oregon reported results from…

On Jan. 24, 2025, The South Dakota Department of Health reported the death of a child due to…

On Jan. 23, 2025, the World Health Organization (WHO) announced that following a nearly century-long effort, Georgia has…

On Jan. 22, 2025, the U.S. Department of Agriculture (USDA) Animal and Plant Health Inspection Service (APHIS) has…

On Jan. 22, 2025, researchers have identified chronic wasting disease (CWD) prions in raw, cooked, and cured meat…

On Jan. 20, 2025, the White House announced the United States will exit the World Health Organization (WHO)….
On Jan. 20, 2025, the World Health Organization (WHO) announced that Tanzania has confirmed an outbreak of Marburg…
On Jan. 20, 2025, a large international 68-country survey of 71,922 respondents found that in most countries, most…

On Jan. 17, 2025, scientists from the National Institute of Allergy and Infectious Diseases (NIAID) reported that an…
On Jan. 17, 2025, scientists from Scripps Research Institute reported finding that some HIV vaccination can lead to…
On Jan. 17, 2025, The U.S. government announced it had awarded Moderna $590 million to advance the development…

On Jan. 17, 2025, the U.S. Centers for Disease Control and Prevention (CDC) reported that eleven pediatric deaths…

On Jan. 16, 2025, Shionogi Inc. has been awarded a $375 million project agreement through the Rapid Response…

On Jan. 15, 2025, officials from Lincoln Park Zoo in Chicago reported that testing had confirmed the highly pathogenic…