One Case of Measles Confirmed in a Kansas Resident
On Mar. 13, 2025, The Kansas Department of Health and Environment (KDHE) and the Stevens County Health Department…

On Mar. 13, 2025, The Kansas Department of Health and Environment (KDHE) and the Stevens County Health Department…

On Mar. 12, 2025, the Animal and Plant Health Inspection Service (APHIS) confirmed the presence of highly pathogenic…

On Mar. 11, 2025, the New York State Department of Health today announced the first case of measles…

On Mar. 11, 2025, researchers from Texas A&M University School of Public Health report findings show significant links…

On Mar. 11, 2025, in an excerpt from his book Booster Shots (Penguin Random House), pediatrician and infectious…

On Mar. 11, 2025, the Oklahoma State Department of Health (OSDH) reported the first two measles cases in…

On Mar. 8, 2025, the World Health Organization (WHO) announced that since the outbreak of Sudan virus disease…

On Mar. 7, 2025, the Texas Department of State Health Services reported updated information about an outbreak of…
On Mar. 6, 2025, the New Mexico Department of Health (NMDOH) confirms that a deceased resident of Lea…
On Mar. 5, 2025, the World Health Organization (WHO) announced In the past two decades, tuberculosis (TB) prevention,…

On Mar. 4, 2025, the Government of Alberta announced it had contributed $100 million in capital funding to…
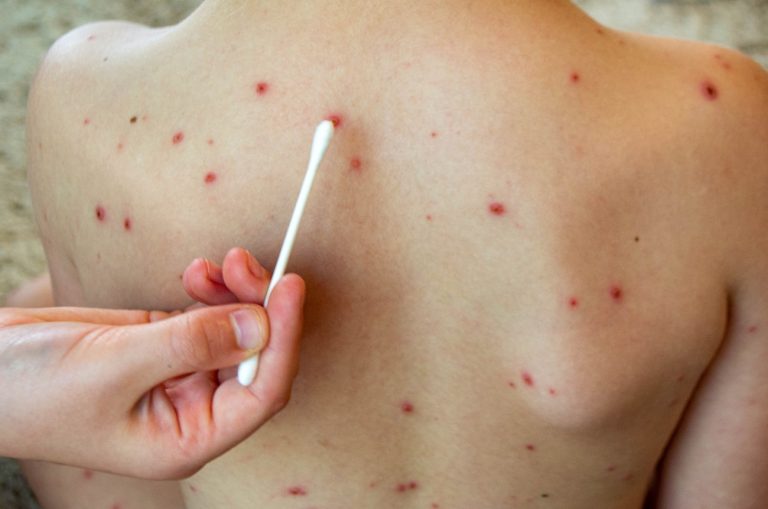
On Mar. 4, 2025, the Texas Department of State Health Services reported an outbreak of measles in the…

On Mar. 3, 2025, LifeScienceHistory.com: Where history is made daily announces the release of a rewritten hit rock…

On Mar. 3, 2025, Revvity announced the launch of EUROIMMUN’s CE-marked Anti-Measles Virus ELISA 2.0 (IgG) to support…

On Feb. 28, 2025, the World Health Organization (WHO) announced announced the recommendations for the viral composition of…
On Feb. 27, 2025, the U.S. Centers for Disease Control and Prevention (CDC) announced that it is investigating…
On Feb. 27, 2025, a new government report adds to evidence that the HPV vaccine, once called dangerous…

On Feb. 27, 2025, research from Oregon State University (OSU) showed that wild birds can account for much…

On Feb. 26, 2025, the U.S. Department of Agriculture (USDA0 announced a $1 billion-dollar comprehensive strategy to curb…

On Feb. 26, 2025, the Texas Department of State Health Services reported the first death from measles in…

In Feb. 26, 2025, Eli Lilly announced that it plans to bolster its domestic medicine production across therapeutic…
On Feb. 24, 2025, the Texas Department of State Health Services reported an outbreak of measles in centered…

On Feb. 18, 2025, a research expedition led by Antonio Alcamí at the Severo Ochoa Molecular Biology Center…

On Feb. 18, 2025, Hologic announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance…

On Feb. 14, 2025, Zoetis announced that the U.S. Department of Agriculture (USDA), Center for Veterinary Biologics (CVB)…

On Feb. 14, 2025, the World Health Organization (WHO) announced that a cumulative of two confirmed and eight…

On Feb. 14, 2025, the Texas Department of State Health Services reported an outbreak of measles in the…

Feb. 14, 2025, the Wyoming Department of Health (WDH) reported that the state’s first case of H5N1 avian…

On Feb. 13, 2025, public health officials from the U.S. Centers for Disease Control and Prevention (CDC) reported…

On Feb. 13, 2025, Ralph L. Abraham, MD, the Louisiana Surgeon General announced that the state will no…