asian history month
Asian American and Pacific Islander History Month celebrates the scientific accomplishments of several firsts, from the first chemo…

Asian American and Pacific Islander History Month celebrates the scientific accomplishments of several firsts, from the first chemo…

On Nov. 15, 2023, the U.S. Food and Drug Administration granted marketing authorization to LetsGetChecked for the Simple…
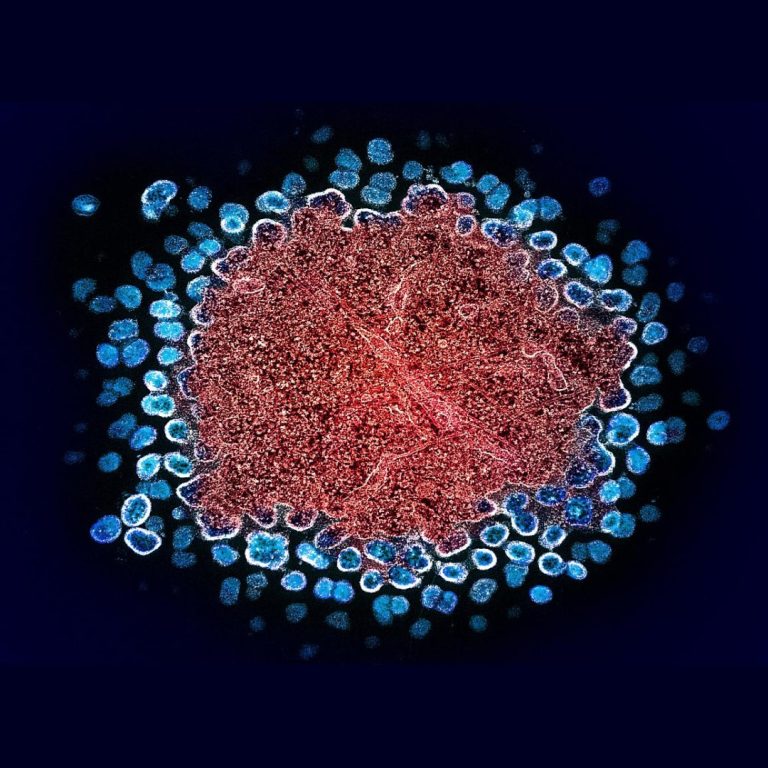
On Sept. 20, 2023, the National Institutes of Health announced that a trial of a preventive HIV vaccine…

On Aug. 24, 2023, researchers at City of Hope announced were awarded $32.3 million from the California Institute…

On Aug. 7, 2023, the United Kingdom Health and Security Agency announced the Vaccine Development and Evaluation Centre…

On Jul. 27, 2023, the California Institute for Regenerative Medicine (CIRM) awarded $50.1 million to fund clinical-stage research…
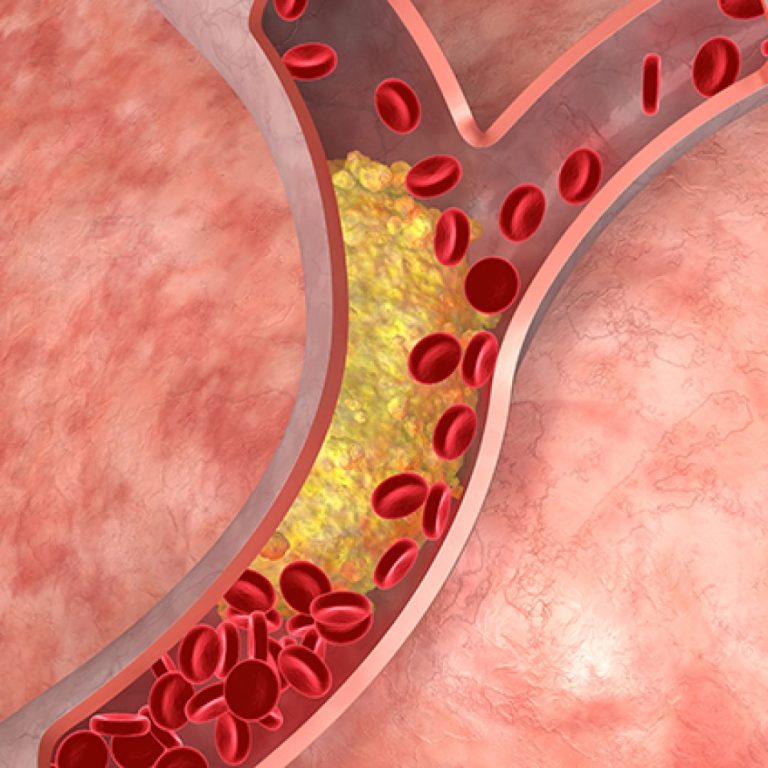
On Apr. 11, 2023, a National Institutes of Health (NIH) clinical trial was stopped early because a daily…
On Feb. 21, 2023, Gilead Sciences announced positive data from three retrospective real-world studies which demonstrated that initiation…
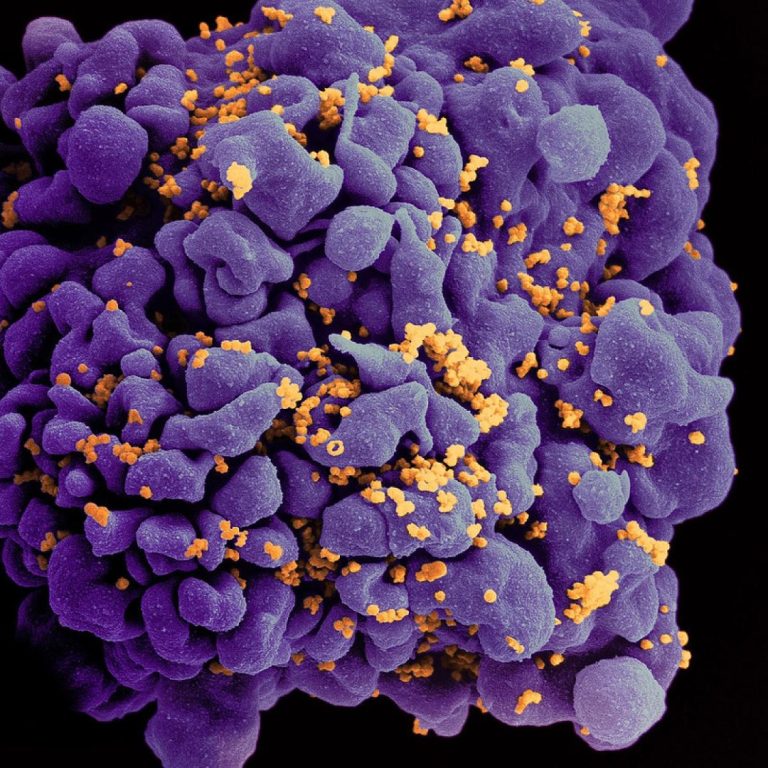
On Feb. 15, 2023, it was reported that a 53-year-old man in Germany had become at least the…

On Jan. 18, 2023, the National Institutes of Health (NIH) announced that an investigational HIV vaccine regimen tested…
On Dec. 22, 2022, Gilead Sciences announced that Sunlenca (lenacapavir), in combination with other antiretroviral(s) (ARV), had been…

On Dec. 2, 2022, in a multi-center collaboration led by Scripps Research, scientists announced they have achieved promising…

On Nov. 1, 2022, the Medicines Control Authority of Zimbabwe announced that it had approved the use of…
On Oct. 27, 2022, the World Health Organization reported that an estimated 10.6 million people fell ill with…
On Oct. 24, 2022, the National Institutes of Health announced that starting antiretroviral treatment (ART) early in the…
On Oct. 20, 2022, the National Institutes of Health announced that a three-dose course of the hepatitis B…

On Jul. 28, 2022, the World Health Organization (WHO) released new guidelines for the use of long-acting injectable…
On Jul. 27, 2022, City of Hope announced that a 66-year-old man who was diagnosed with HIV in…

On May 18, 2022, Moderna and the nonprofit scientific research organization IAVI announced that the first participant screenings…

On Apr. 7, 2022, Moderna and IAVI announced a collaboration to employ mRNA technology to meet the challenge…

On Mar. 18, 2002, Johnson & Johnson announced an agreement to acquire all of the assets of Tibotec-Virco…

On Mar. 14, 2022, researchers from the National Institute of Allergy and Infectious Diseases (NIAID) announced they had…
On Feb. 15, 2022, a woman with HIV who received a cord blood stem cell transplant to treat…

On Jan. 24, 2022, Anixa Biosciences announced that the company and its partner, MolGenie, had synthesized a compound…

On Jan 5, 2022, Hologic the addition of the Aptimaᆴ SARS-CoV-2 assay to its Global Access Initiative, a…

On Oct. 29, 2021, research led by scientists at the National Institutes of Health announced they had identified…

On Oct. 18, 2021, Gilead Sciences announced that the Food and Drug Administration had approved a new low-dose…

On Oct. 13, 2021, the World Health Organization (WHO) honoured the late Henrietta Lacks with a WHO Director-General’s…

On Oct. 12, 2021, National Resilience announced a strategic collaboration with Children’s Hospital of Philadelphia (CHOP) to implement…

On Sept. 22, 2021, the Breakthrough Prize Foundation and its founding sponsors announced the 2022 winners of the…