U.S. completes withdrawal from World Health Organization
On Jan. 22, 2026, The U.S. has finalized its withdrawal from the World Health Organization (WHO), one year…

On Jan. 22, 2026, The U.S. has finalized its withdrawal from the World Health Organization (WHO), one year…
On Dec. 18, 2025, the World Health Organization (WHO) announced it has validated Brazil for the elimination of…

On Nov. 21, 2025, the U.S. Centers for Disease Control and Prevention (CDC) announced it has instructed staffers…

On Nov. 20, 2025, the National Institutes of Health (NIH) has canceled funding for at least 383 clinical…
On Sept. 24, 2025, Indian drugmakers Dr Reddy’s Laboratories and Hetero Labs announced they will sell generic versions…

On Aug. 23, 2025, award-winning HIV scientist Shan Liang, a tenured associate professor at the Washington University School…

On Jul. 17, 2025, a new world-leading biosecurity centre in Essex has been announced that will protect the…
On Jul. 15, 2025, in 2024, 89% of infants globally – about 115 million – received at least…
On Jul. 14, 2025, The World Health Organization (WHO) released new guidelines recommending the use of injectable lenacapavir…

On Jul. 14, 2025, researchers at Burnet Institute, in collaboration with Gavi, the Vaccine Alliance, have provided the…

On Jun. 25, 2025, in the midst of a multi-state measles outbreak, a poll by Harvard T.H. Chan…

On Jun. 25, 2025, the U.S. will announced it will no longer contribute funding to Gavi, a global…

On Jun. 18, 2025, Gilead Sciences announced that the U.S. Food and Drug Administration (FDA) has approved Yeztugo®…

On Jun. 12, 2025, U.S. Health Secretary Robert Kennedy Jr. named eight members to serve on a key…
On May, 27, 2025, an Ohio State University (OSU) study has revealed the biological secret to the Zika…

On May 8, 2025, Bill Gates announced that he has decided to give away his money back to…

On May 1, 2025, the U.S. National Institutes of Health (NIH), the world’s largest funder of biomedical research,…

On Apr. 21, 2025, LifeScienceHistory.com published the cartoon: “The National Institutes of Health (NIH) and U.S. Healthcare in…
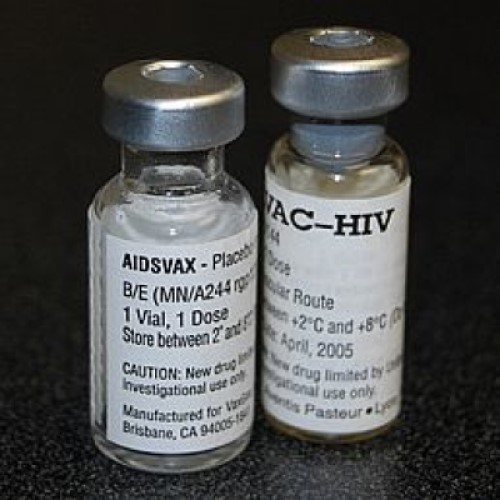
On Apr. 17, 2025, a study was published that shows the United States spent roughly US$12 billion on…

On Apr. 11, 2025, Africa’s top public health institution announced it is planning to tap more funds from…
On Apr. 2, 2025, A team of Northwestern University scientists spanning disciplines announced they have developed new technology…

On Mar. 27, 2025, a Nature survey of more than 1,200 scientists, three-quarters of the total respondents are…

On Mar. 25. 2025, the UK Health Security Agency (UKHSA) published its view on the pathogen families that…

On Feb. 5, 2025, the proposed dismantling of the U.S. Agency for International Development (USAID) could disrupt clinical…

On Jan. 17, 2025, scientists from Scripps Research Institute reported finding that some HIV vaccination can lead to…
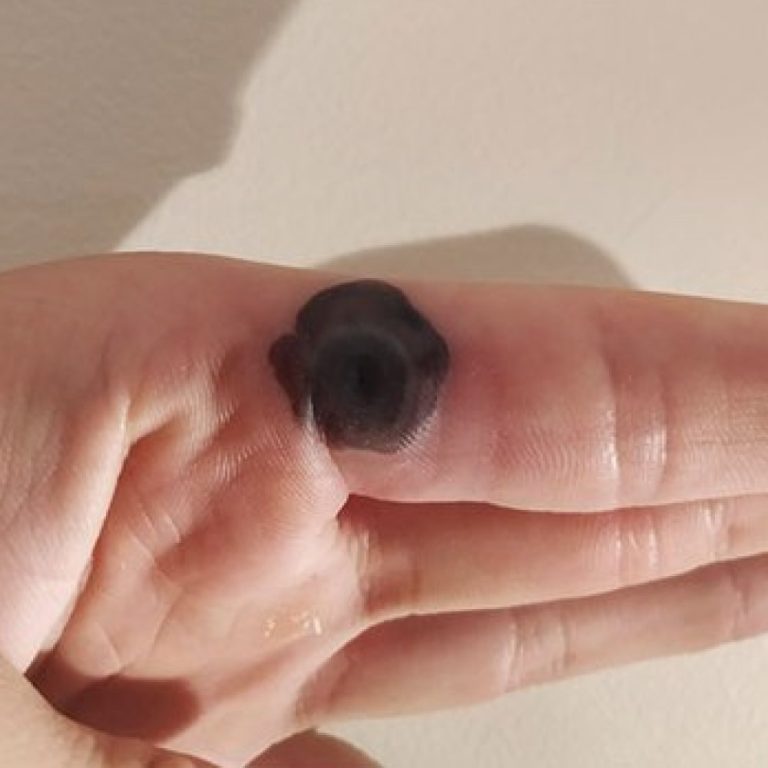
On Dec. 5, 2024, the World Health Organization (WHO) announced that as of December 1, 20 countries have…

On Nov. 26, 2024, the the United Nations reported that fewer people contracted HIV last year than at…
On Nov. 19, 2024, the World Health Organization (WHO) announced it had granted Emergency Use Listing (EUL) to Japan-based…

On Nov. 5, 2024, a World Health Organization (WHO) study published in eBioMedicine named 17 pathogens that regularly…
On Jul. 25, 2024, the World Health Organization (WHO) announced during the 25th International AIDS Conference, being held…