U.S. FDA issued low-acid food processing regulations after botulism outbreaks from canned foods
On Feb. 17, 1973, the U.S. Food and Drug Administration (FDA) announced the recall of 29,500 institutional-size cans of…

On Feb. 17, 1973, the U.S. Food and Drug Administration (FDA) announced the recall of 29,500 institutional-size cans of…

On Oct. 27, 1972, the Consumer Product Safety Commission (CPSA) was enacted by the U.S. Congress. The CPSA…

On Jul. 1, 1972, the Regulation of Biologics–including serums, vaccines, and blood products–was transferred from the NIH to…

In 1972, the Division of Biologics Standards was transferred from the National Institutes of Health (NIH) to the U.S….

In 1972, the Food and Drug Administration’s (FDA) new Bureau of Biologics began to regulate all 7000 U.S….

On Sept. 29, 1971, the artificial sweetener saccharin, included in FDA’s original GRAS list, was removed from the…

On May 17, 1971, The Public Health Service’s Bureau of Radiological Health was transferred to the FDA.

In 1971, the Public Health Service (PHS) Bureau of Radiological Health was transferred to U.S. Food and Drug…

On Feb. 27, 1970, in Upjohn v. Finch the Court of Appeals upheld enforcement of the 1962 drug…
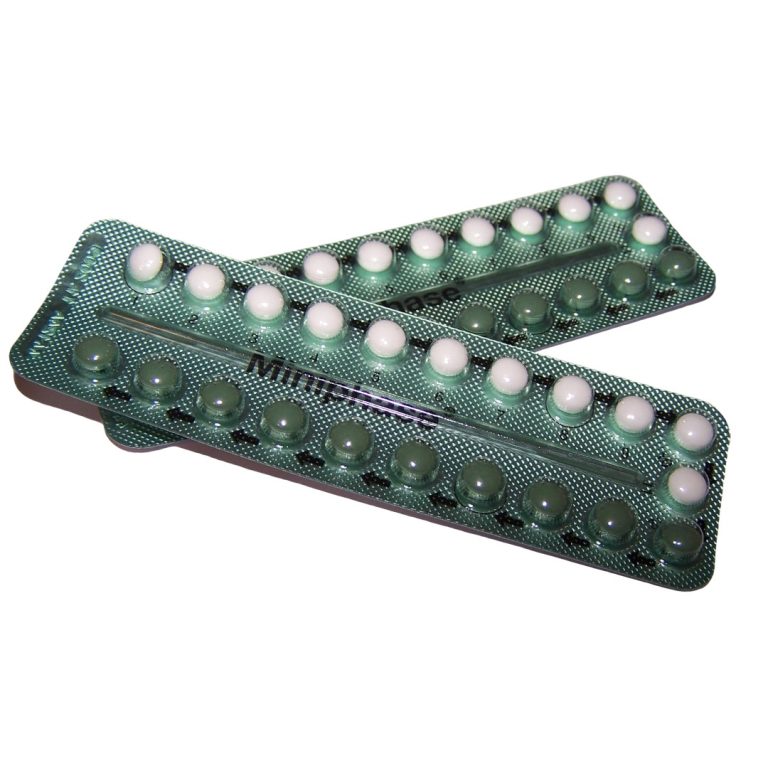
In 1970, the U.S. Food and Drug Administration (FDA) required the first patient package insert: oral contraceptives must contain…

On Dec. 13, 1969, Charles Edwards, M.D., becomes FDA commissioner.

On Dec. 2, 1969, the White House Conference on Food, Nutrition, and Health (WHC) was a seminal event…

On Sept. 4, 1969, the FDA issued a report that called birth control pills safe, despite a slight…

In 1969, the U.S. Food and Drug Administration (FDA) began administering Sanitation Programs for milk, shellfish, food service, and…
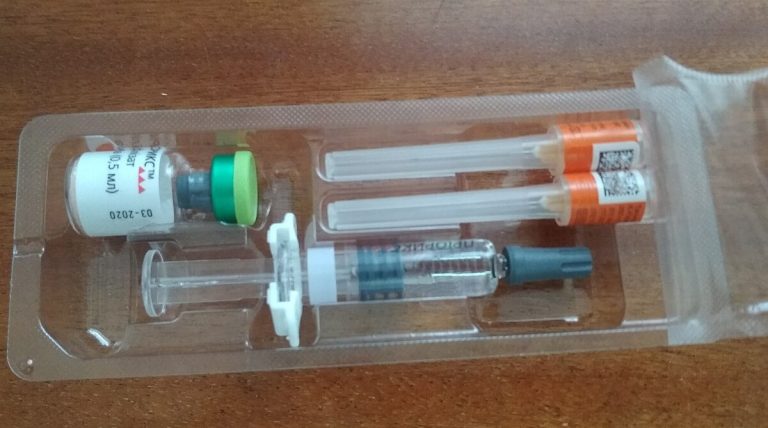
In 1969, live, attenuated rubella vaccines were first licensed in the U.S., and a vaccination program was established…
On Nov. 26, 1968, the U.S. Food and Drug Administration (FDA) licensed a second live, further attenuated measles…
On Apr. 7, 1968, the FDA Bureau of Drug Abuse Control and Treasury Department Bureau of Narcotics were…

In March 1968, a reorganization of federal health programs placed the U.S. Food and Drug Administration (FDA) in…

In 1968, the U.S. Food and Drug Administration (FDA) formed the Drug Efficacy Study Implementation (DESI) to implement recommendations…

In 1968, Medtronic annual sales skyrocketed to more than $12 million, with the company reporting net income in…
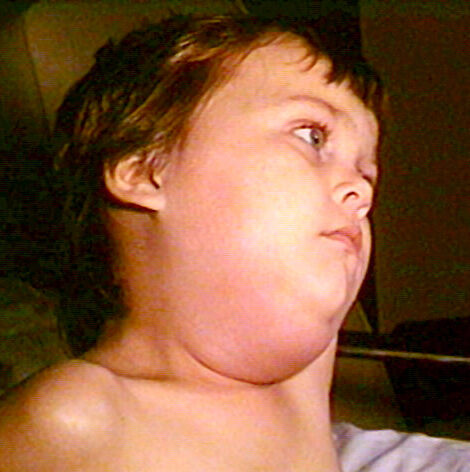
On Dec. 28, 1967, the U.S. Food and Drug Administration (FDA) approved Mercks mumps virus vaccine live (MumpsVax)….

In 1967 the Fair Packaging and Labeling Act (FPLA or Act), enacted by the U.S. Congress directs the…

In 1967, Medtronic introduced two “on-demand” pacemakers, designed to avoid competition between paced beats and the patient’s own…

On Nov. 3, 1966, the Child Protection Act was passed by the U.S. Congress. The bill enlarged the…

In 1966, the Drug Efficacy Study of the National Research Council’s Division of Medical Sciences, which was tasked…
In 1966, the U.S. Food and Drug Administration (FDA) licensed amantadine (marketed as Symmetrel) to Du Pont, a…

On Aug. 30, 1964, the U.S. Food and Drug Administration (FDA) requested help in removing “X-33 Water Repellent”…

In 1964, the anticancer drug melphalan (L-PAM) was approved by the U.S. Food and Drug Administration.
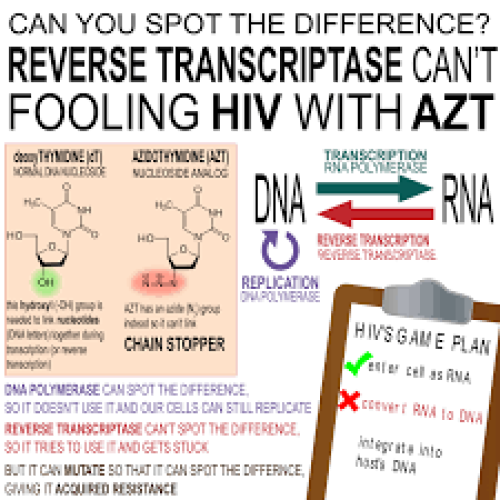
In 1964, the anticancer drug Azidothymidine (AZT) was synthesized in Michigan Cancer Foundation’s chemistry lab by Jerome Horwitz,…

On Jul. 10, 1963, the U.S. FDA approved vincristine, a sister drug to vinblastine. The drug was established…