Qureator Achieves World’s First FDA IND Approval Using Only Human Vascularized Organoid Efficacy Data
On Oct. 27, 2025, Qureator announced a major milestone, confirming a fundamental shift in drug development under the…

On Oct. 27, 2025, Qureator announced a major milestone, confirming a fundamental shift in drug development under the…
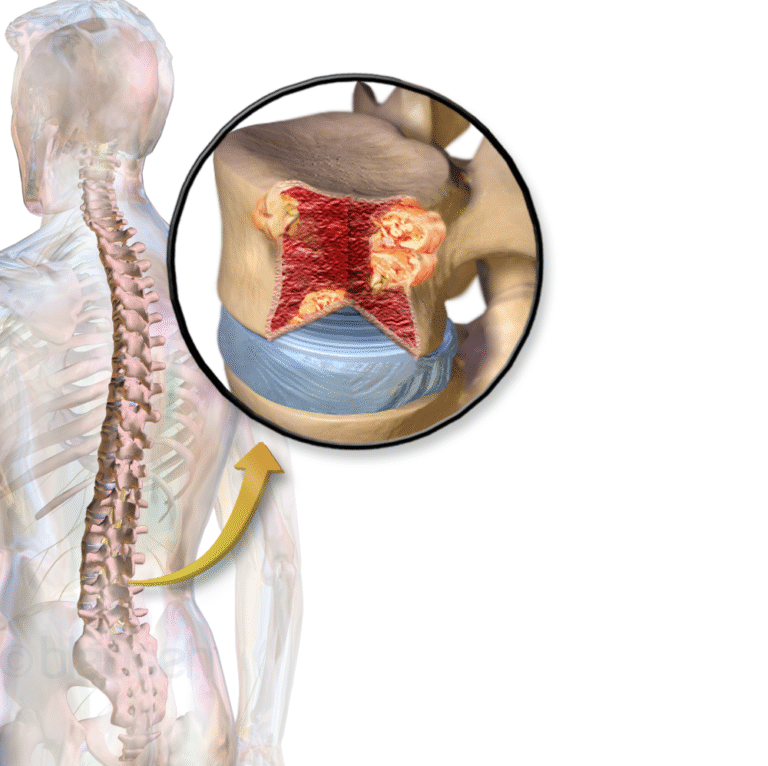
On Oct. 23, 2025, GSK announced the US Food and Drug Administration (FDA) has approved Blenrep (belantamab mafodotin-blmf)…

On Oct. 20, 2025, Glaukos announced today the U.S. Food and Drug Administration (FDA) approved its Epioxa™ HD…

On Oct. 17, 2025, Novo Nordisk announced that the US Food and Drug Administration (FDA) has approved Rybelsus®, the only oral GLP-1…

On Oct. 16, 2025, the Food and Drug Administration (FDA) announced the first round of experimental drugs that…

On Oct. 13, 2025, the U.S. Food and Drug Administration (FDA) announces it has cleared the Elecsys pTau181…

On Oct. 9, 2025, Boehringer Ingelheim’s JASCAYD® (nerandomilast) tablets has been approved by the U.S. Food and Drug…

On Oct. 9, 2025, Celltrion announced announced that the U.S. Food and Drug Administration (FDA) has approved EYDENZELT® (aflibercept-boav), biosimilar…

On Oct. 3, 2025, the U.S. Food and Drug Administration (FDA) announced a new pilot prioritization program for…
On Oct. 2, 2025, the U.S. Food and Drug Administration (FDA) approved Evita Solution’s generic version of the…

On Sept. 22, 2025, the U.S. Food and Drug Administration (FDA) announced it h initiated the approval of…

On Sept. 15, 2025, Corstasis Therapeutics announced that the U.S. Food and Drug Administration (FDA) has approved ENBUMYST™…

On Sept. 15, 2025, the U.S. Department of Agriculture (USDA) Animal and Plant Health Inspection Service (APHIS) National…

On Sept. 8, 2025, Pfizer and BioNTech announced positive topline results from an ongoing Phase 3 clinical trial…

On Sept. 4, 2025, roughly 24 hours after the launch of the West Coast Health Alliance, Hawaii is…

On Sept. 4, 2025, Novavax announced a milestone payment from Takeda has been triggered by regulatory approval for Novavax’s…
On Sept. 3, 2025, California Governor Gavin Newsom, Oregon Governor Tina Kotek, and Washington Governor Bob Ferguson announced…

On Aug. 27, 2025, Novavax announced that the U.S. Food and Drug Administration (FDA) has approved the Nuvaxovid™ 2025-2026…
On Aug. 25, 2025, shares of French drugmaker Valneva slumped more than 20% after the U.S. Food and…

On Aug. 18, 2025, Vanda Pharmaceuticals announced that it secured a landmark victory over the U.S. Food and…

On Aug. 18, 2025, Novo Nordisk announced the launch of a new offer that enables self-paying, eligible, type…

On Aug. 15, 2025, Novo Nordisk announced that the US Food and Drug Administration (FDA) has approved a…

On Aug. 15, 2025, Precigen announced that the US Food and Drug Administration (FDA) has approved PAPZIMEOS™ (zopapogene imadenovec-drba)…

On Aug. 8, 2025, the U.S.Department of Health and Human Services (HHS) announced that it was winding down…
On Aug. 6, 2025, the Tacoma-Pierce County Health Department (TPCHD) reported an East Pierce County, Washington woman who…

On Jul. 31, 2025, Novo Nordisk announced that the US Food and Drug Administration (FDA) approved Alhemo® (concizumab-mtci)…

On Jul. 21, 2025, Biogen announced it intends to invest an additional $2 billion in its existing manufacturing…

On Jul. 18, 2025, the U.S. Food and Drug Administration (FDA) announced it has placed Sarepta Therapeutics investigational…
On Jul. 10, 2025, Moderna announced that the U.S. Food and Drug Administration (FDA) has approved the supplemental…

On Jul. 9, 2025, Eli Lilly announced that the U.S. Food and Drug Administration (FDA) has approved a…