FDA launched the Digital Health Center of Excellence
On Sept. 22, 2020, the FDA announced it had launched the Digital Health Center of Excellence within the…

On Sept. 22, 2020, the FDA announced it had launched the Digital Health Center of Excellence within the…

On Sept. 18, 2020, Roche announced the launch of its Elecsysᆴ Anti-SARS-CoV-2 S antibody test for markets accepting…
On Sept. 16, 2020, AXIM Biotechnologies announced that it had filed an Emergency Use Authorization (EUA) application with…

On Sept. 15, 2020, the FDA published comparative performance data for some authorized COVID-19 molecular diagnostic tests. The…

On Sept. 15, 2020, the FDA awarded a $5.4 million research contract to the University of Liverpool and…

On Sept. 14, 2020, Vaxart announced that the FDA had completed its review of the Companyメs Investigational New…

On Sept. 12, 2020, Pfizer and BioNTech announced that they had submitted an amended protocol to the FDA…
On Sept. 11, 2020, Todos Medical announced that the FDA has added the TODOS 2019-nCoV RT-qPCR Detection Kit…

On Sept. 10, 2020, Accelerate Diagnostics and BioCheck announced that the FDA had issued an Emergency Use Authorization…
On Sept. 9, 2020, Pfizer and BioNTech announced that they had submitted an amended protocol to the FDA…
On Sept. 10, 2020, GlaxoSmithKline and Innoviva announced the U.S. Food and Drug Administration (FDA) had approved a…

On Sept. 8, 2020, INOVIO announced that Thermo Fisher Scientific, the world leader in serving science, had signed…
On Sept. 3, 2020, OraSure Technologies announced that its ORAcollect-ᄋRNA (OR-100) collection device was included along with other…

On Sept. 3, 2020, Roche announced that the cobas SARS-CoV-2 & Influenza A/B Test for use on the…

On Sept. 1, 2020, Roche announced FDA approval for the cobasᆴ HIV-1/HIV-2 Qualitative Test for use on the…

On Sept. 1, 2020, City of Hope announced that it was investigating an innovative treatment for cancer patients…

On Aug. 31, 2020, T2 Biosystems announced that the FDA had issued an Emergency Use Authorization (EUA) for…

On Aug. 28, 2020, the FDA issued an emergency use authorization (EUA) to Yale School of Public Health…

On Aug. 28, 2020, Gilead Sciences announced the FDA expanded the Emergency Use Authorization (EUA) enabling use of…

On Aug. 27, 2020, Laurent Pharmaceuticals announced that it had received approval from the U.S. Food and Drug…

On Aug. 26, 2020, Abbott announced the FDA had issued Emergency Use Authorization (EUA) for its BinaxNOWル COVID-19…

On Aug. 24, 2020, XBiotech announced that the U.S. Food and Drug Administration (FDA) issued an emergency use…

On Aug. 23, 2020, the FDA issued an emergency use authorization (EUA) for investigational convalescent plasma for the…
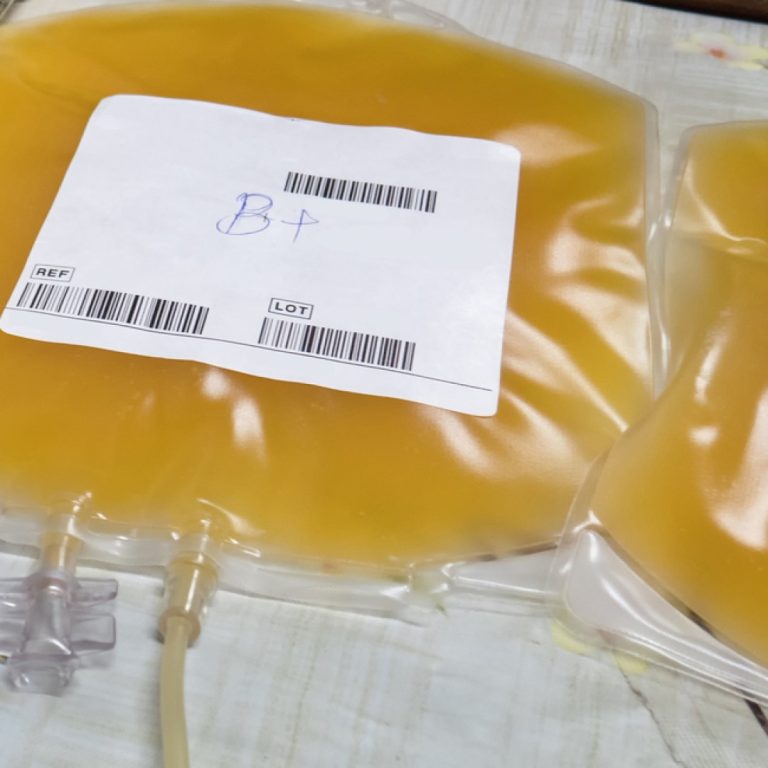
On Aug. 23, 2020, the Mayo Clinic announced that with the FDA Emergency Use Authorization of convalescent plasma,…

On Aug. 19, 2020, Accelerate Diagnostics and BioCheck announced that the FDA had issued an Emergency Use Authorization…

On Aug. 17, 2020, the U.S. Food and Drug Administration (FDA) approved Genentech’s Enspryng (satralizumab-mwge) for the treatment…

On Aug. 15, 2020, the FDA issued an emergency use authorization (EUA) to Yale School of Public Health…

On Aug. 15, 2020, a saliva-based laboratory diagnostic test developed by researchers at the Yale School of Public…

On Aug. 12, 2020, Baxter announced it had received Emergency Use Authorizations (EUAs) from the FDA for the…

On Aug. 12, 2020, the FDA granted accelerated approval to NS Pharma’s Viltepso (viltolarsen) injection for the treatment…