U.S. FDA approved TICOVACル, Pfizerメs tick-borne encephalitis vaccine
On Aug. 13, 2021, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved TICOVACル (tick-borne…

On Aug. 13, 2021, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved TICOVACル (tick-borne…

On Aug. 13, 2021, Merck announced that the U.S. Food and Drug Administration (FDA) had approved WELIREG, an…

On Aug. 13, 2021, Rigel Pharma announced that the U.S. Food and Drug Administration (FDA) had informed the…
On Aug. 13, 2021, Moderna announced that the Food and Drug Administration (FDA) had approved an update to…

On Jul. 30, 2021, Regeneron announced that the FDA had updated the Emergency Use Authorization (EUA) for the…

On Jul. 29, 2021, Incyte announced the U.S. Food and Drug Administration (FDA) had broadened the Emergency Use…

On Jul. 29, 2021, BOTOX(R) Allergan, an AbbVie company, announced that the U.S. Food and Drug Administration (FDA)…

On Jul. 29, 2021, Emergent BioSolutions announced that the FDA had begun allowing Emergent’s Bayview manufacturing facility to…
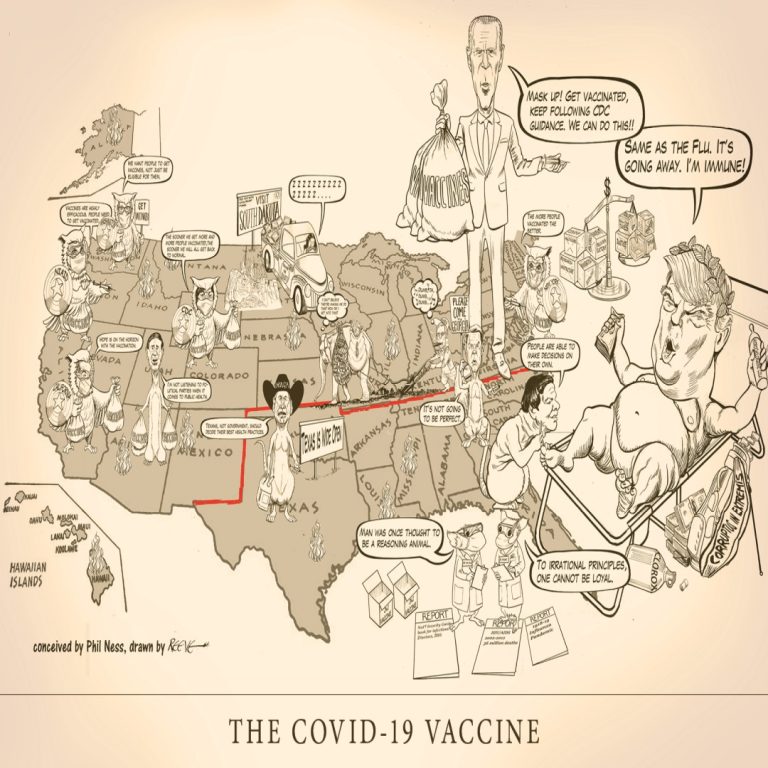
Cast of Characters: Donald Trump, President | Mike Pence, Vice President | Anthony S. Fauci, MD, Director, National…
On Jul. 16, 2021, Merck announced the U.S. Food and Drug Administration (FDA) had approved VAXNEUVANCE (Pneumococcal 15-valent…

On Jul. 16, 2021, Pfizer and BioNTech announced that the U.S. Food and Drug Administration (FDA) had granted…

On Jul. 13, 2021, the Food and Drug Administration (FDA) revisions to the vaccine recipient and vaccination provider…

On Jul. 9, 2021, the Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) to Ortho-Clinical…

On Jul. 2, 2021, the Food and Drug Administration (FDA) authorized the use, under the emergency use authorization…

On Jun. 28, 2021, Meridian Bioscience announced that it had re-submitted its application for Emergency Use Authorization (EUA)…
On Jun. 25, 2021, Roche announced that the Food and Drug Administration (FDA) has issued an Emergency Use…

On Jun. 23, 2021, Biogen and Eisai announced that the U.S. Food and Drug Administration (FDA) had granted…

On Jun. 11, 2021, Emergent BioSolutions announced that two batches of COVID-19 vaccine manufactured by at its Baltimore…

On Jun. 11, 2021, Quidel announced it had received an amended Emergency Use Authorization (EUA) from the U.S….

On Jun. 11, 2021, The FDA issued an emergency use authorization (EUA) for the Janssen COVID-19 vaccine, two…

On Jun. 10, 2021, Moderna announced that it had requested an emergency use authorization (EUA) for its COVID-19…

On Jun. 9, 2021, Vertex Pharmaceuticals announced the U.S. Food and Drug Administration (FDA) approved expanded use of…

On Jun. 8, 2021, the University of Oxford announced that the RECOVERY trial found aspirin does not improve…

On Jun. 7, 2021, Biogen and Eisai announced that the U.S. Food and Drug Administration (FDA) had granted…

On Jun. 7, 2021, OraSure Technologies announced that it had received Emergency Use Authorization (EUA) from the FDA…

On Jun. 4, 2021, Chimerix announced that the U.S. Food and Drug Administration (FDA) had granted TEMBEXAᆴ (brincidofovir)…

On Jun. 4, 2021, the U.S. Food and Drug Administration announced it had approved Novo Nordisk’s Wegovy (semaglutide)…

On Jun. 4, 2021, Regeneron announced the U.S. Food and Drug Administration (FDA) updated the Emergency Use Authorization…

On Jun. 3, 2021, Innovation Pharma announced that it had achieved full patient enrollment in its randomized, double-blind,…

On Jun. 2, 2021, RELIEF THERAPEUTICS reported that its collaboration partner, NRx Pharmaceuticals, announced that it had submitted…