Moderna announced FDA authorization of booster dose of COVID-19 vaccine in the U.S.
On Oct. 20, 2021, Moderna announced that the U.S. Food and Drug Administration (FDA) had authorized for emergency…

On Oct. 20, 2021, Moderna announced that the U.S. Food and Drug Administration (FDA) had authorized for emergency…

On Oct. 20, 2021, Johnson & Johnson announced the U.S. Food and Drug Administration (FDA) had issued Emergency…
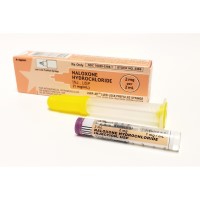
On Oct. 18, 2021, the Food and Drug Administration approved ZIMHI (naloxone hydrochloride) injection as an additional option…

On Oct. 18, 2021, Gilead Sciences announced that the Food and Drug Administration had approved a new low-dose…

On Oct. 15, 2021, Roche announced that the U.S. Food and Drug Administration (FDA) had approved Tecentriq (atezolizumab)…

On Oct. 14, 2021, Moderna confirmed that the U.S. Food and Drug Administration’s (FDA) Vaccines and Related Biological…

On Oct. 14, 2021, Regeneron announced that the U.S. Food and Drug Administration (FDA) had accepted for priority…

On Oct. 11, 2021, Merck and Ridgeback Biotherapeutics announced that Merck had submitted an Emergency Use Authorization (EUA)…
On Oct. 7, 2021, PerkinElmer announced that the U.S. Food and Drug Administration (FDA) had issued Emergency Use…

On Oct. 5, 2021, PerkinElmer announced that the U.S. Food and Drug Administration had provided Emergency Use Authorization…

On Oct. 5, 2021, Johnson & Johnson announced it had submitted data to the U.S. Food and Drug…

On Oct. 1, 2021, Merck and Ridgeback Biotherapeutics announced that molnupiravir (MK-4482, EIDD-2801), an investigational oral antiviral medicine,…

On Oct. 1, 2021, Kite, a Gilead Company, announced the U.S. Food and Drug Administration had granted approval…

On Sept. 30 2021, Siemens Healthineers announced the Food and Drug Administration (FDA) 510 (k) clearance of the…

On Sept. 29, 2021, Pfizer and BioNTech announced they had submitted data to the U.S. Food and Drug…
On Sept. 28, 2021, Pfizer and BioNTech announced they had submitted data to the U.S. Food and Drug…

On Sept. 23, 2021, Pfizer and BioNTech announced that the U.S. Food and Drug Administration (FDA) has authorized…
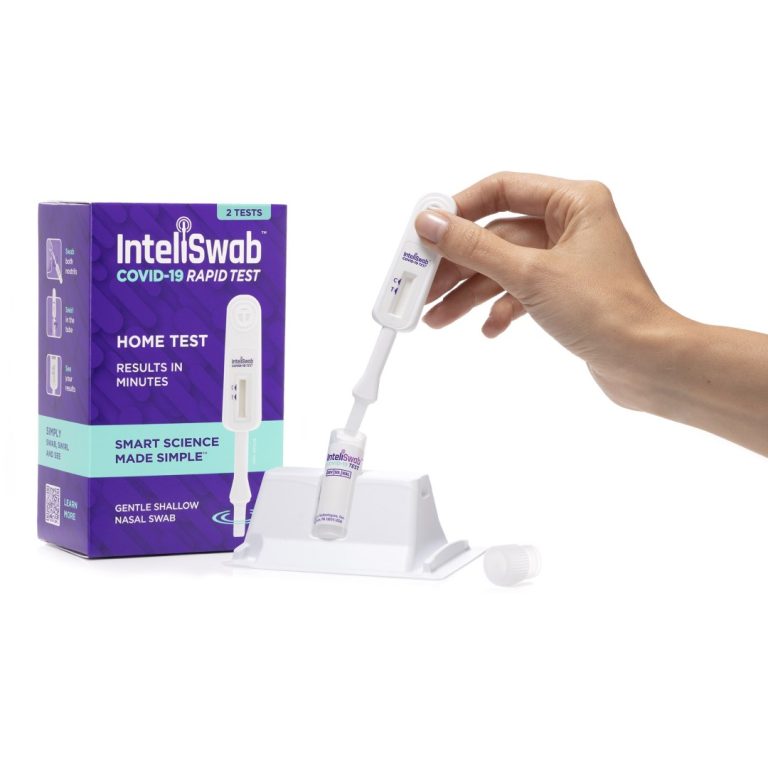
On Sept. 23, 2021, OraSure Technologies and the Biomedical Advanced Research Development Authority (BARDA) announced up to $13.6…

On Sept. 15, 2021, InBios announced that it had received Emergency Use Authorization from the U.S. Food and…

On Sept. 9, 2021, Humanigen announced the U.S. FDA had declined its request for emergency use authorization of…

On Sept. 8, 2021, AIM ImmunoTech announced that is had submitted a Pre-Investigational New Drug application (Pre-IND) to…

On Sept. 6, 2021, Pfizer and BioNTech announced that they had submitted a variation to the European Medicines…

On Sept. 1, 2021, Moderna announced it had initiated its submission to the U.S. Food and Drug Administration…

On Aug. 25, 2021, Moderna announced it had completed the rolling submission process for its Biologics License Application…

On Aug. 25, 2021, BD (Becton, Dickinson) announced the U.S. Food and Drug Administration (FDA) had issued an…

On Aug. 25, 2021, Pfizer and BioNTech announced the initiation of a supplemental Biologics License Application (sBLA) to…

On Aug. 23, 2021, the U.S. Food and Drug Administration approved the first COVID-19 vaccine. The vaccine, known…

On Aug. 23, 2021, Pfizer and BioNTech announced that the U.S. Food and Drug Administration (FDA) approved the…

On Aug. 16, 2021, Pfizer and BioNTech announced that they had submitted Phase 1 data to the U.S….

On Aug. 16, 2021, ADMA Biologics announced that it had received U.S. Food and Drug Administration approval for…