Moderna received full U.S. FDA approval for COVID-19 vaccine Spikevax
On Jan. 31, 2022, Moderna announced the U.S. Food and Drug Administration (FDA) had approved the Biologics License…

On Jan. 31, 2022, Moderna announced the U.S. Food and Drug Administration (FDA) had approved the Biologics License…

On Jan. 31, 2022, Novavax announced that it had submitted a request to the U.S Food and Drug…
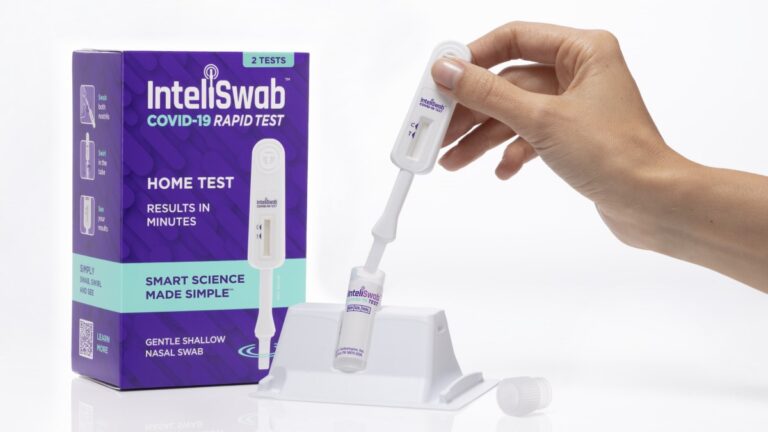
On Jan. 31, 2022, OraSure Technologies announced that its InteliSwab COVID-19 rapid tests had been authorized by the…

On Jan. 31, 2022, Veru announced that the U.S. Food and Drug Administration (FDA) had granted Fast Track…

On Jan. 26, 2022, the NIH announced that adults who had previously received a full regimen of any…
On Jan. 20, 2022, Gilead Sciences announced that the U.S. Food and Drug Administration had granted expedited approval…

On Jan. 12, 2022, Novavax and SK bioscience, a vaccine business subsidiary of Korea-based SK Group, announced that…

On Jan. 11, 2022, Pfizer and BioNTech announced that the U.S. Food and Drug Administration had expanded the…

On Jan. 10, 2022, Novartis Molecular Partners announced that Part A of the EMPATHY clinical trial that compared…

On Jan 5, 2022, NRx Pharmaceuticals announced that it had submitted an application for Emergency Use Authorization (EUA)…

On Jan. 3, 2022, Pfizer and BioNTech announced that the U.S. Food and Drug Administration had expanded the…

On Dec. 27, 2021, the U.S. Food and Drug Administration authorized an over-the-counter (OTC) COVID-19 antigen test, the…

Our Heroes and Remembrance illustration has Maurice Ralph Hilleman and John Enders, pioneering developers of common vaccines, and…

On Dec. 23, 2021, Merck and Ridgeback Biotherapeutics announced that the U.S. Food and Drug Administration (FDA) had…

On Dec. 22, 2021, Pfizer announced that the U.S. Food and Drug Administration (FDA) had authorized the emergency…

On Dec. 9, 2021, CytoDyn announced that it had submitted a Phase 3, randomized, double blind, placebo controlled…

On Dec. 9, 2021, Pfizer and BioNTech announced that the U.S. Food and Drug Administration (FDA) had expanded…

On Dec. 5, 2021, Roche announced that its planned to launch the SARS-CoV-2 & Flu A/B Rapid Antigen…
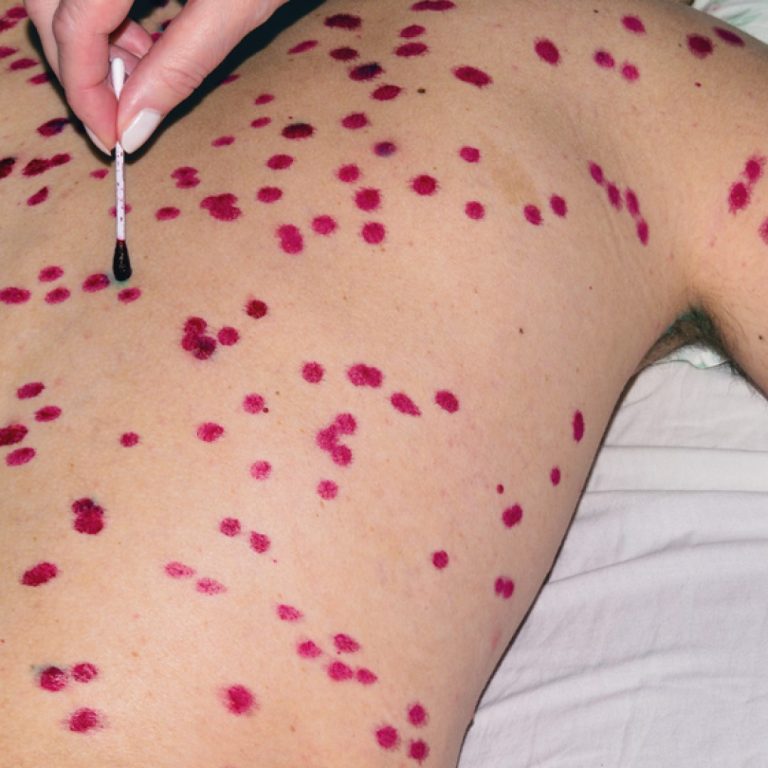
On Dec. 1, 2021, SIGA Technologies announced that Health Canada had approved oral TPOXX (tecovirimat) as an extraordinary…
On Nov. 30, 2021, Inovio Pharma announced the company was rapidly moving to evaluate its COVID-19 DNA vaccine…

On Nov. 24, 2021, Johnson & Johnson announced the U.S. Food and Drug Administration (FDA) had issued Emergency…

On Nov. 22, 2021, the U.S. Food and Drug Administration (FDA) authorized another over-the-counter (OTC) COVID-19 antigen test. The…

On Nov. 19, 2021, the U.S. Food and Drug Administration (FDA) announced that it had extended the emergency…

On Nov. 19, 2021, Pfizer and BioNTech announced that the U.S. Food and Drug Administration (FDA) had expanded…
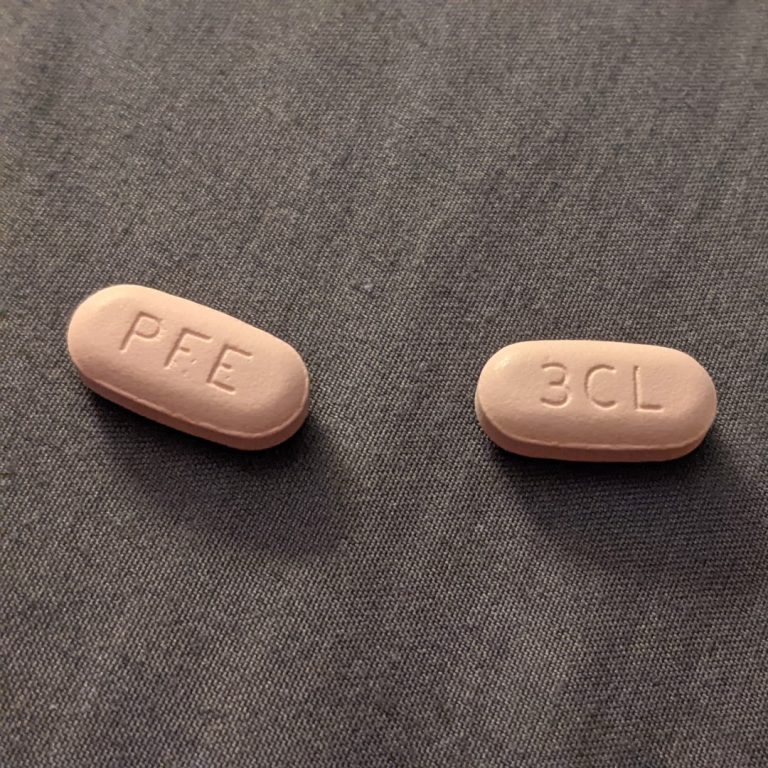
On Nov. 18, 2021, Pfizer announced an agreement with the U.S. government to supply 10 million treatment courses…

On Nov. 17, 2021, Zosano Pharma announced that the Philippine Food and Drug Administration had granted emergency use…

On Nov. 17, 2021, GlaxoSmithKline and Vir Biotechnology announced U.S. government contracts totalling approximately $1 billion (USD) to…

On Nov. 16, 2021, Pfizer announced it had submitted an Emergency Use Authorization (EUA) of its investigational oral…

On Nov. 11, 2021, Innovation Pharma reported topline results from the Companyメs Phase 2 clinical trial of Brilacidin…

On Nov. 10, 2021, Meridian Bioscience announced that their Revogeneᆴ SARS-CoV-2 assay was granted Emergency Use Authorization by…