Navy base in Italy reports case of West Nile virus
On Sept. 3, 2025, Navy hospital here is warning people to take precautions against West Nile virus after…

On Sept. 3, 2025, Navy hospital here is warning people to take precautions against West Nile virus after…

On Sept. 2, 2025, Amgen announced plans to invest more than $600 million in a new, state-of-the-art center…

On Sept. 2, 2025, Arrowhead Pharmaceuticals announced a global licensing and collaboration agreement with Novartis for ARO-SNCA, Arrowhead’s…
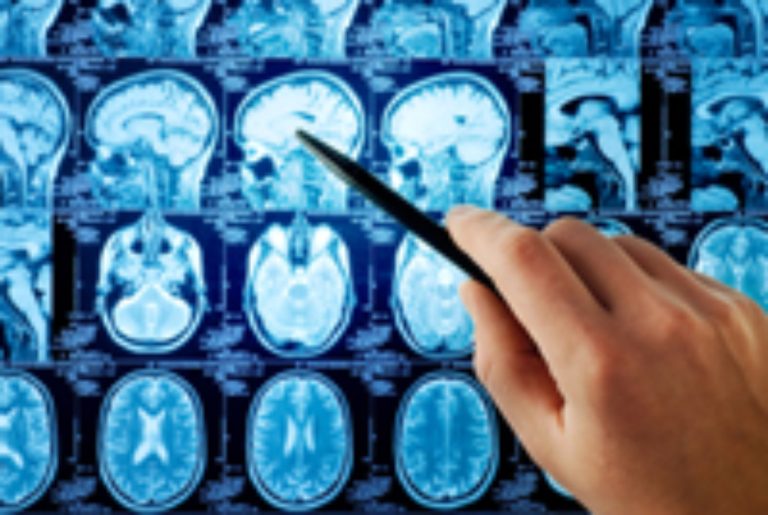
On Sept. 1, 2025, a multi-institutional study suggests that typically classifiication of meningiomas by appearances may be deceiving…
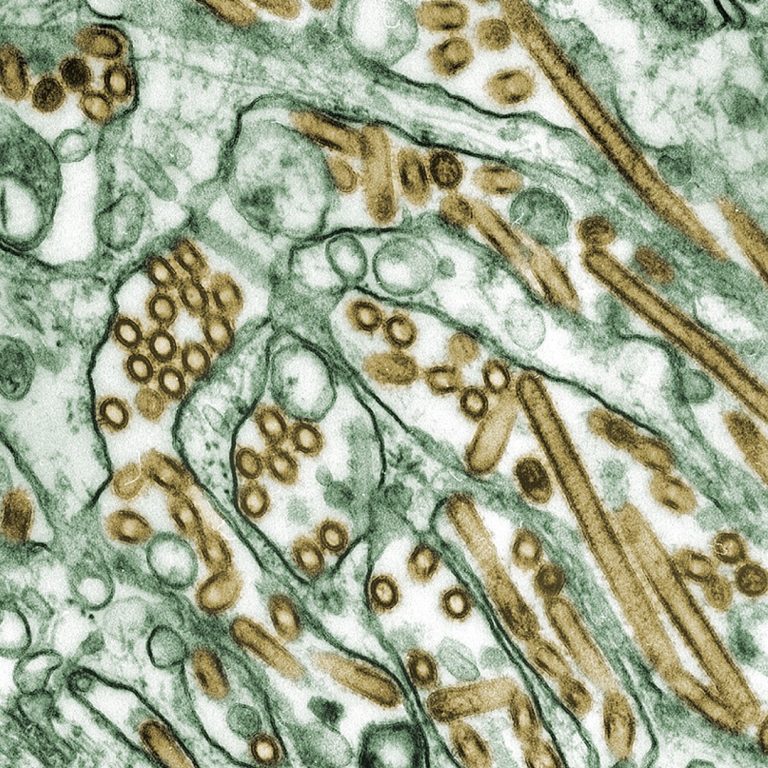
On Aug. 28, 2025, scientists at MIT’s Computer Science and Artificial Intelligence Laboratory (CSAIL) and the MIT Abdul…

On Aug. 27, 2025, Mexico has recorded 5,086 cases of flesh-eating screwworm in animals as of August 17,…
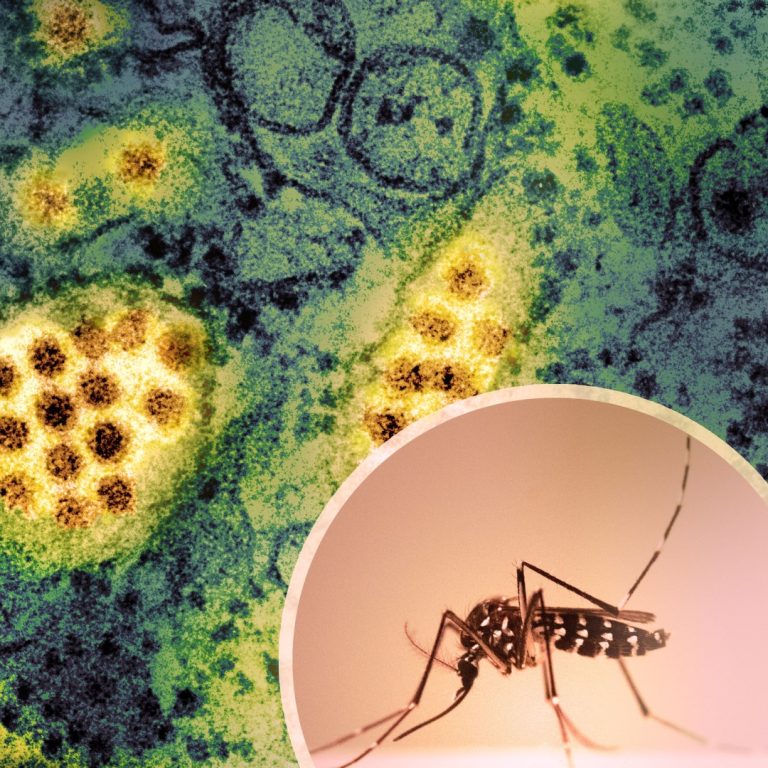
On Aug. 26, 2025, health officials in Ashland, Oregon reported that six Aedes aegypti mosquitoes were found in…

On Aug. 25, 2025, Genentech, a member of the Roche Group broke ground on its newest U.S. manufacturing site…

On Aug. 25, 2025, C2N Diagnostics (C2N) announced lower age limit addresses increasing calls from clinicians seeking access…

On Aug. 24, 2025, the U.S. Department of Health and Human Services reported the first human case in…
On Aug. 21, 2025, the Mississippi State Department of Health (MSDH) declared a public health emergency in response…
On Aug. 20, 2025, The Cancer Prevention and Research Institute of Texas (CPRIT) announced the approval of 15…

On Aug. 20, 2025, two studies led by Johns Hopkins Kimmel Cancer Center, Ludwig Center, and Johns Hopkins Whiting School of…

On Aug. 19, 2025, health officials have uncovered another death in connection with a Legionnaires’ disease outbreak in…

On Aug. 18, 2025, Lewis and Clark Public Health (LCPH) officlials in Montana have confirmed a single case…

On Aug. 18, 2025, the Texas Department of State Health Services is reporting the end of this year’s…

On Aug. 18, 2025, the World Health Organization announced Nepal has eliminated rubella as a public health problem,…

On Aug. 14, 2025, Phil and Penny Knight announced a record-breaking $2 billion gift to the Oregon Health…

On Aug. 14, 2025, with help from artificial intelligence, researchers from Massachusetts Institute of Technology (MIT) announced they…

On Aug. 14, 2025, the California Department of Public Health (CDPH) is reminding California residents and visitors that…
On Aug. 14, 2025, a fourth person has died in connection with a Legionnaires’ disease outbreak in New…

On Aug. 12, 2025, Powassan virus (POWV), a type of flavivirus closely related to the Zika and Dengue…

On Aug. 11, 2025, University of Washington (UW) researchers announced the results of testing the efficacy of several…

On Aug. 10, 2025, computer scientists at ETH Zurich announced they have developed a digital tool capable of…
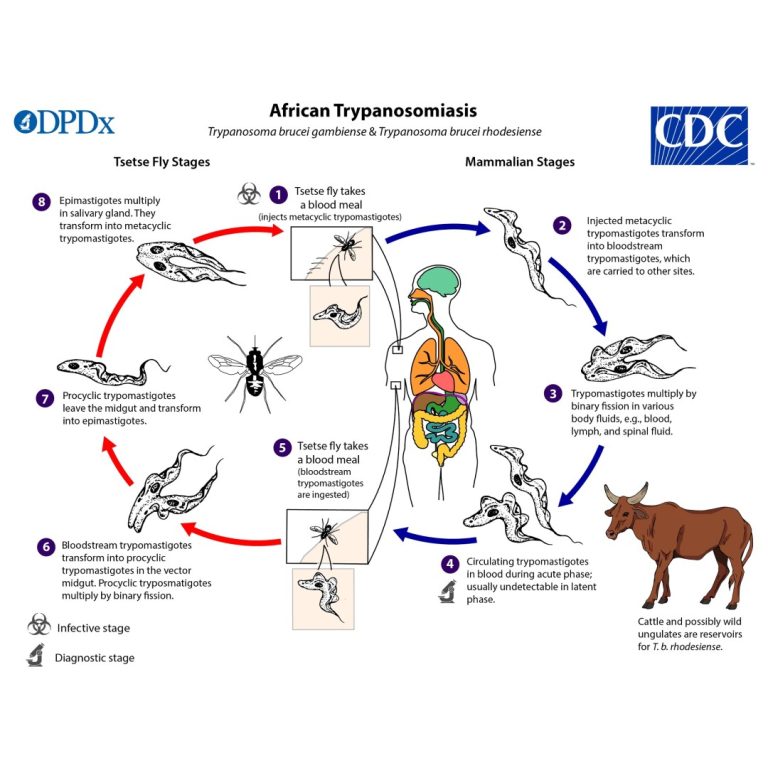
On Aug. 8, 2025, the World Health Organization (WHO) has validated Kenya as having eliminated human African trypanosomiasis…
On Aug. 8, 2025, experts in mosquito-borne infectious diseases are warning against excessive insect eradication campaigns as cities…
On Aug. 6, 2025, the Tacoma-Pierce County Health Department (TPCHD) reported an East Pierce County, Washington woman who…

On Aug. 4, 2025, The Gates Foundation announced a $2.5 billion commitment through 2030 to accelerate research and…

On Aug. 4, 2025, UK Biobank announced it had reached a historic milestone – completing 100,000 whole-body imaging…

On Aug. 4, 2025, The Florida Department of Health said on Monday that there have been 21 cases…