FDA approved first topical gene therapy for treatment of wounds in patients with dystrophic epidermolysis bullosa
On May 19, 2023, the U.S. Food and Drug Administration (FDA) approved Krystal Biotech’s Vyjuvek, a herpes-simplex virus…
On May 19, 2023, the U.S. Food and Drug Administration (FDA) approved Krystal Biotech’s Vyjuvek, a herpes-simplex virus…

On May 15, 2023, Brazil, the world’s top chicken exporter, for the first time confirmed Highly Pathogenic Avian…

On May 9, 2023, the U.S. Preventive Services Task Force (USPSTF) recommended that all women get screened for…
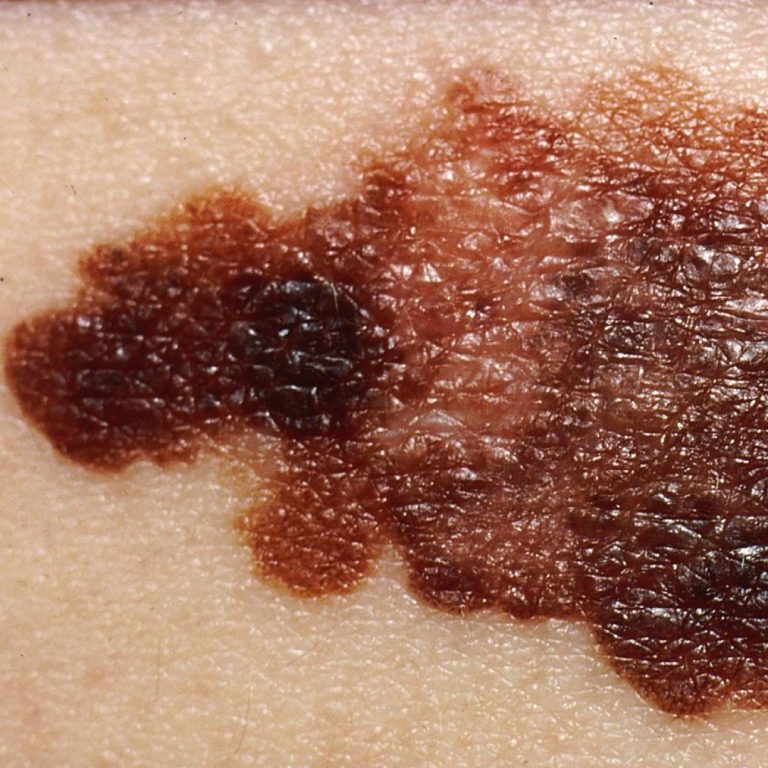
On May 1, 2023, an Oregon Health Sciences University (OHSU) dermatologist and a multi-disciplinary team confirmed that a…

On Apr. 27, 2023, the Zoonomia Project team announced in in a special issue of Science, that had…

On Apr. 27, 2023, researchers at Cornell University announced they had used ancient DNA extraction and analysis to…

On Apr. 24, 2023, the U.S. Food and Drug Administration (FDA) authorized Status COVID-19 Antigen Rapid Test for…

On Apr. 23, 2023, the most comprehensive state-by-state analysis of the impacts of COVID-19 across the USA, published…

On Apr. 18, 2023, the U.S. Preventive Services Task Force (USPSTF) published a final recommendation statement on screening…

On Apr. 17, 2023, a new initiative led by Jennifer Doudna and Jill Banfield at the Innovative Genomics…

On Apr. 14, 2023, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization for the…

On Apr. 13, 2023, in an enormous leap forward in the understanding of Parkinson’s disease (PD), researchers announced…

On Apr. 4, 2023, National Institutes of Health (NIH) funded research was announced from the University of Rochester…
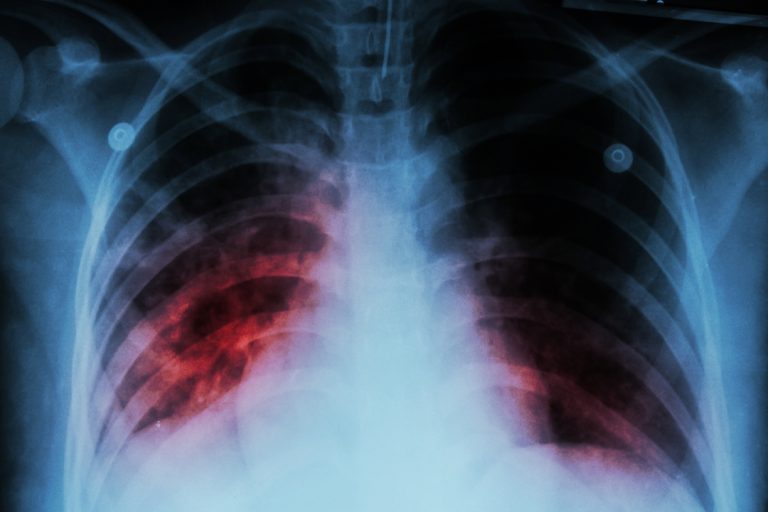
On Mar. 24, 2023, the U.S. Centers for Disease Control and Prevention (CDC) announced that Tuberculosis (TB) in…

On Mar. 23, 2023, the Fred Hutchinson Cancer Center (FHCRC) announced a new building in South Lake Union…

On Mar. 17, 2023, the Indonesia Ministry of Health notified the World Health Organization (WHO) of the detection…

On Mar. 15, 2023, the UK Animal and Plant Health Agency reported that the carcesses of Common dolphins…
On Mar. 15, 2023, scientists at Texas A&M announced that a research study: ‘RNA interference is essential to…

On Mar. 14, 2023, the Agricultural and Livestock Service in Chile confirmed the identification of the first case…

On Mar. 9, 2023, the U.S. Food and Drug Administration (FDA) announced that it had published updates to…
On Feb. 28, 2023, Public Health Delta & Menominee Counties (PHDM) was alerted to three cases of atypical…

On Feb. 28, 2023, a team from the University of Sydney reported that a flu virus found in…
On Feb. 24, 2023, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for…

On Feb. 22, 2023, the Centers for Disease Control and Prevention (CDC) reported that the 2022-2023 flu vaccines…

On Feb. 15, 2023, the Cancer Prevention and Research Institute of Texas (CPRIT) announced a major step forward…

On Feb. 14, 2023, the Pacific Economic Development Agency of Canada (PacifiCan), announced $14.5 million in funding for…

On Feb. 10, 2023, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for…

On Feb. 9, 2023, the National Institutes of Health (NIH) announced that a new large-scale genetic analysis found…

On Feb. 8, 2023, as bird flu continues to circle the globe, a new report suggested that the…
On Feb. 8, 2023, BD announced that it had received Emergency Use Authorization from the U.S. Food and…