FDA Approved Drug with New Mechanism of Action for Treatment of Schizophrenia
On Sept. 26, 2024, the U.S. Food and Drug Administration (FDA) approved Bristol-Myers Squibb’s Cobenfy (xanomeline and trospium…

On Sept. 26, 2024, the U.S. Food and Drug Administration (FDA) approved Bristol-Myers Squibb’s Cobenfy (xanomeline and trospium…

On Sept. 24, 2024, the Mark O. Hatfield Clinical Research Center at the National Institutes of Health (NIH)…

On Sept. 24, 2024, researchers at the National Institutes of Health (NIH) reported results of a study that…

On Sept. 23, 2024, researchers from the Wellcome Sanger Institute and their collaborators at GeneDx, announced results of…

On Sept. 23, 2024, researchers from Columbia University reported that a study of Children born during the first…

On Sept. 20, 2024, a research team led by scientists at the University of California, Riverside, announced they…
On Sept. 19, 2024, Oxitec announced it had launched Sparks™, a new commercial platform to accelerate the global…
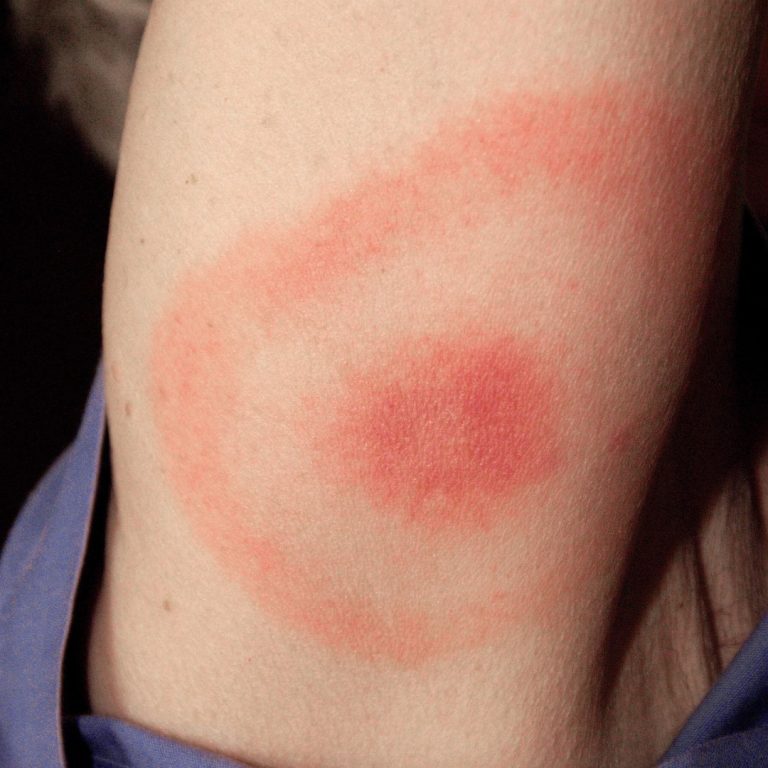
On Sept. 19, 2024, the nonprofit organization Focus on Lyme and Aces Diagnostics announced a breakthrough in Lyme…

On Sept. 19, 2024, a U.S. population-based birth cohort study was released that showed that 53.4% of babies…

On Sept. 19, 2024, an international team of researchers announced a study that genetically traced the epicenter of…

On Sept. 18, 2024, a study published in The Lancel reported that here has been a rapid rise…
On Sept. 18, 2024, researchers from the Wellcome Sanger Institute and their collaborators reported that they had identified…

On Sept. 17, 2024, in landmark research, scientists at The University of Texas Health Science Center at San…

On Sept. 17, 2024, a team of researchers from the Food Packaging Forum announced that a peer-reviewed scientific…

On Sept. 17, 2024, researchers from Tufts University announced they had received a $20.7 million grant from the…

On Sept. 16, 2024, researchers from the National Health Service Blood and Transplant (Bristol), NHSBT’s International Blood Group…

On Sept. 16, 2024, a study by the Global Research on Antimicrobial Resistance (GRAM) Project reported that antimicrobial…
On Sept. 16, 2024, A 24-year-old student has died from the Nipah virus in the southern Indian state…

On Sept. 12, 2024, the Finnish Institute for Health and Welfare (THL) reported that Poliovirus had been found…

On Sep. 11, 2024, the Translational Genomics Research Institute (TGen), part of City of Hope, announced the launch…

On Sep. 11, 2024, researchers at UTHealth Houston and Baylor College of Medicine reported that Avian influenza A(H5N1)…
On Sep. 10, 2024, researchers at Weill Cornell Medicine in Qatar announced they had created a free-to-access online…

On Sept. 10, 2024, a study, led by the University of Edinburgh, was the first to explore the…
On Sep. 6, 2024, researchers from the Wellcome Sanger Institute, University College London (UCL), and the University of…

On Sept. 5, 2024, the U.S. Department of Justice announced it had awarded nearly $8 million to cities,…
On Sep. 4, 2024, the World Health Organization (WHO) published global cholera statistics for 2023, that showed an…
On Sept. 3, 2024, a major study led by the UC Davis Comprehensive Cancer Center (UC DAVIS) has…
On Sept. 3, 2024, researchers at NORC at the University of Chicago and George Washington University’s Milken Institute…

On Sep. 3, 2024, the National Cancer Institute (NCI) announced that the latest Cancer Trends Progress Report included…

On Sep. 2, 2024, the World Health Organization (WHO) confirmed that a laboratory-confirmed case of human infection with…