New methods reveal cancer mechanism in ancient genes
On Mar. 3, 2025, researchers at Fred Hutch Cancer Center announced they have discovered an overlooked mechanism driving…

On Mar. 3, 2025, researchers at Fred Hutch Cancer Center announced they have discovered an overlooked mechanism driving…

On Feb. 28, 2025, the World Health Organization (WHO) announced announced the recommendations for the viral composition of…
On Feb. 27, 2025, the U.S. Centers for Disease Control and Prevention (CDC) announced that it is investigating…
On Feb. 27, 2025, a new government report adds to evidence that the HPV vaccine, once called dangerous…

On Feb. 27, 2025, ALK announced that the U.S. Food and Drug Administration (FDA) has approved ALK’s ODACTRA®…

On Feb. 27, 2025, research from Oregon State University (OSU) showed that wild birds can account for much…

On Feb. 26, 2025, the U.S. Department of Agriculture (USDA0 announced a $1 billion-dollar comprehensive strategy to curb…

On Feb. 26, 2025, the Texas Department of State Health Services reported the first death from measles in…

In Feb. 26, 2025, Eli Lilly announced that it plans to bolster its domestic medicine production across therapeutic…
On Feb. 24, 2025, the Texas Department of State Health Services reported an outbreak of measles in centered…

On Feb. 24, 2025, breast cancer is the most common cancer in women worldwide, but survival chances vary…

On Feb. 21, 2025, Novo Nordisk announced that the U.S. Food and Drug Administration (FDA) has determined the…
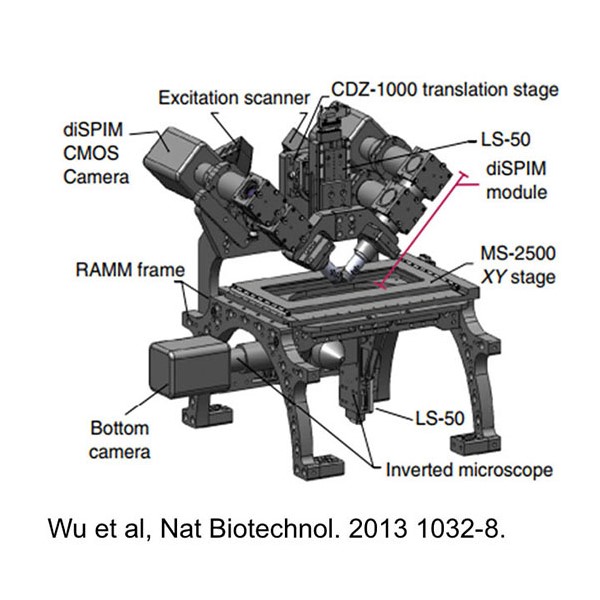
On Feb. 21, 2025, a team of researchers from the Marine Biological Laboratory (MBL) announced a hybrid custom-designed…

On Feb. 20, 2025, researchers from the University of Washington and Seattle Children’s Research Institute announced findings from…

On Feb. 19, 2025, the Cancer Prevention and Research Institute of Texas (CPRIT) announced that it had approved…

On Feb. 19, 2025, Alcon announced the full U.S. commercial availability of Voyager™ DSLT, the first and only…

On Feb. 19, 2025, research from the Texas A&M College of Veterinary Medicine and Biomedical Sciences has discovered…
On Feb. 19, 2025, scientists at St. Jude Children’s Research Hospital report results from a promising new approach…

On Feb. 18, 2025, a research expedition led by Antonio Alcamí at the Severo Ochoa Molecular Biology Center…

On Feb. 18, 2025, Hologic announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance…

On Feb. 18, 2025, scientists from the Institute for Systems Biology announced they have developed a breakthrough method…

On Feb. 17, 2025, Stanford researchers have conducted the first large-scale screen of these inherited changes, called single…

On Feb. 14, 2025, the World Health Organization (WHO) announced that a cumulative of two confirmed and eight…

On Feb. 14, 2025, the Texas Department of State Health Services reported an outbreak of measles in the…

Feb. 14, 2025, the Wyoming Department of Health (WDH) reported that the state’s first case of H5N1 avian…

On Feb. 13, 2025, public health officials from the U.S. Centers for Disease Control and Prevention (CDC) reported…
On Feb. 13, 2025, researchers at Washington University School of Medicine in St. Louis announced a new analysis…
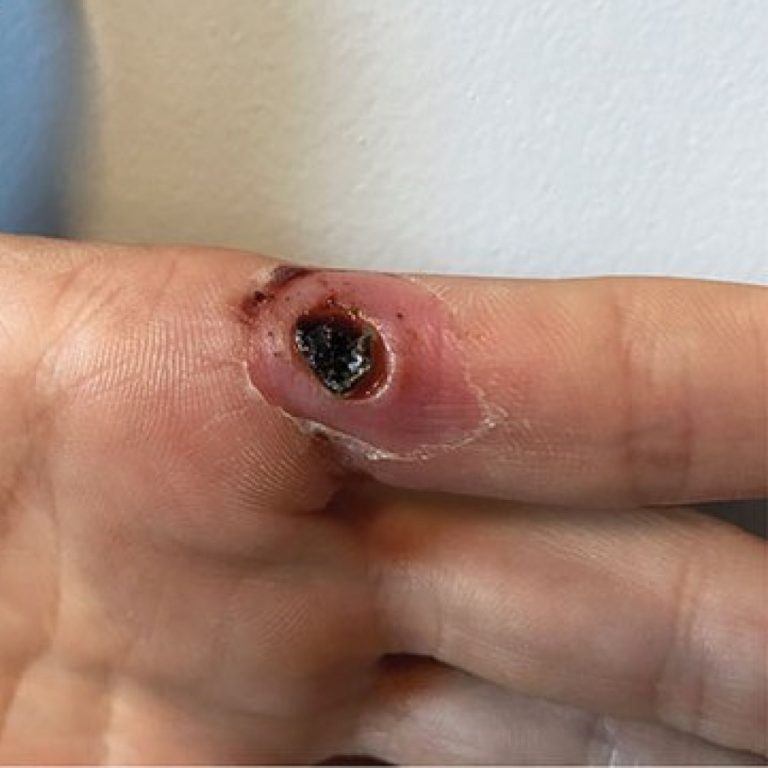
On Feb. 12, 2025, the New York State Department of Health issued a Health Advisory to health care…
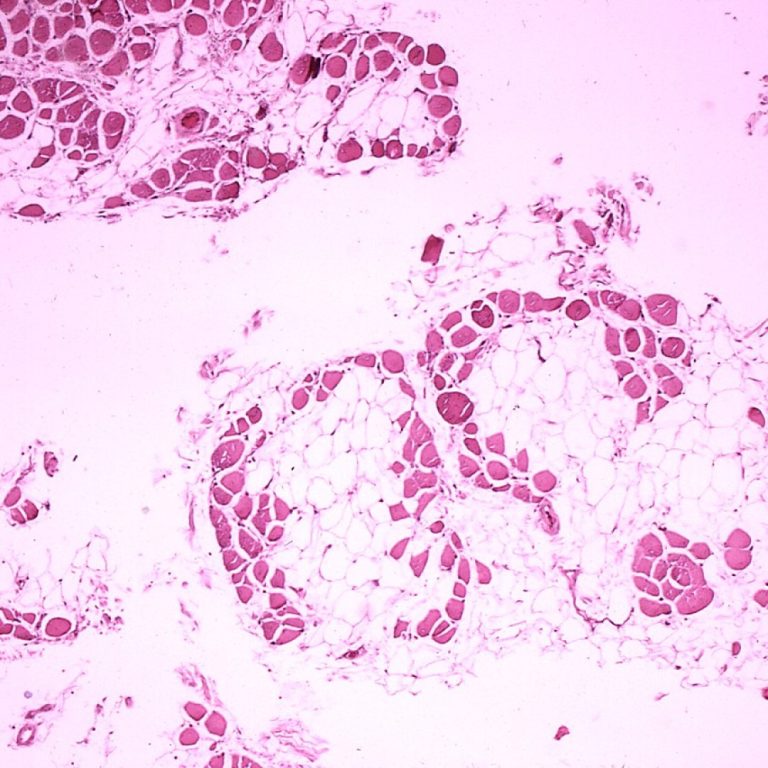
On Feb. 12, 2025, researchers at the Max Planck Institute released a study that could pave the way…

On Feb. 12, 2025, a study led by researchers from the University of Washington announced that a new…