US measles cases soar to 588 so far this year as South Carolina confirms 58 new infections
On Jan. 30, 2026, the U.S. Centers for Disease Control and Prevention (CDC) confirmed 172 new measles cases…

On Jan. 30, 2026, the U.S. Centers for Disease Control and Prevention (CDC) confirmed 172 new measles cases…

On Jan. 29, 2026, in a new study from Sweden, researchers found no statistically significant difference in rates…

On Jan. 27, 2026, South Carolina reported a surge to 789 measles cases, state health data showed, overtaking…

On Jan. 22, 2026, The U.S. has finalized its withdrawal from the World Health Organization (WHO), one year…
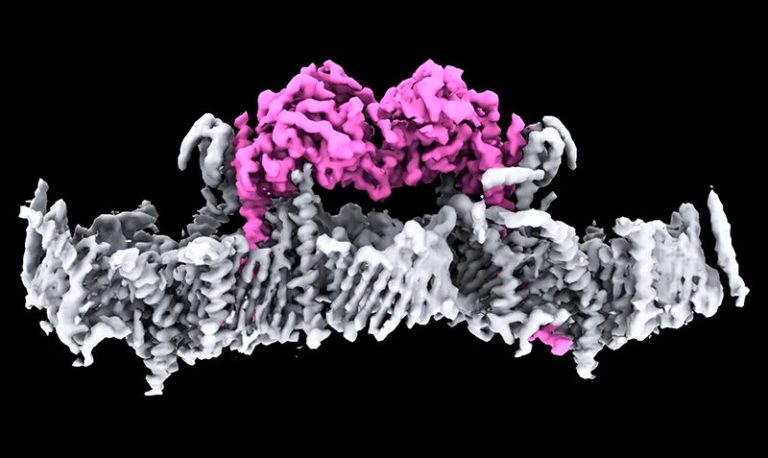
On Jan. 22, 2026, researchers backed by a Knight Initiative for Brain Resilience Catalyst Momentum Award have laid out…
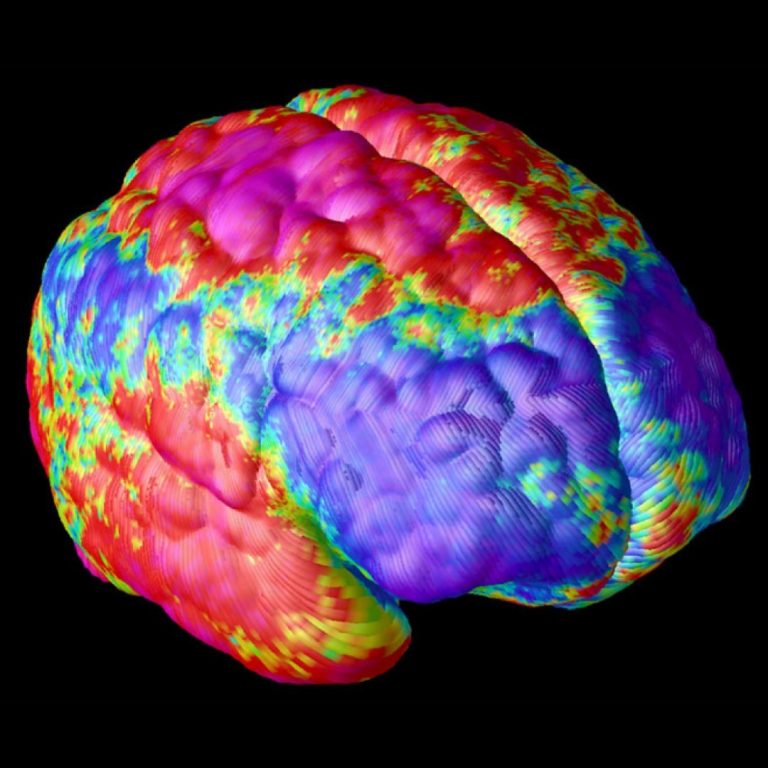
On Jan. 20, 2026, researchers announced they have identified two biomarkers — verbal learning and identifying other peoples’…
On Jan. 15, 2026, the Snohomish County Health Department in Washington has confirmed three new measles cases this…

On Jan. 14, 2026, U.S. healthcare spending rose by 7.2% to $5.3 trillion in 2024 from $4.9 trillion…
On Jan. 14, 2026, a new study published in JAMA Network suggest that health care costs associated with…

On Jan. 13, 2026, collaborating with Google’s top artificial intelligence labs, a team of Yale researchers announced they…

On Jan. 13, 2026, The American Cancer Society (ACS) released Cancer Statistics, 2026, the organization’s annual report on cancer facts and trends. The findings show, for…

On Jan. 8, 2026, Chinese drug makers’ licensing deals more than doubled in 2025 from a year earlier…

On Jan. 8, 2026, a major advance in cell biology has revealed how our cells safeguard their genetic…
On Jan. 8, 2026, researchers reported results from a study on the largest cohort of autobrewery syndrome (ABS)…

On Jan. 7, 2026, the U.S. Food and Drug Administration (FDA) said that it will limit regulation of…

On Jan. 6, 2026, a systematic analysis for the Global Burden of Disease Global study shows that lower…
On Jan. 6, 2026, the U.S. Centers for Disease Control and Prevention (CDC) reported that for the full…

On Jan. 6, 2026, a study led by University of Washington School of Medicine researchers uncovered several new…
On Jan. 5, 2026, researchers at the University of Illinois Urbana-Champaign have developed a groundbreaking new tool that…

On Jan. 1, 2026, the Centers for Disease Control and Prevention (CDC) reported that in September 2024, the…
On Dec. 24, 2025, the World Health Organization (WHO) reported that since the beginning of 2025 and as…
On Dec. 20, 2025, a new study published in the journal BMC Infectious Diseases reported that viral emergence…
On Dec. 18, 2025, the World Health Organization (WHO) announced it has validated Brazil for the elimination of…

On Dec. 18, 2025, new national and state data was released by the Partnership to Fight Chronic Disease…
On Dec. 17, 2025, A survey of Alzheimer’s disease prevalence in Norway confirms earlier estimates and might show…
On Dec. 17, 2025, researchers at the University of Pennsylvania’s (UPenn) School of Engineering and Applied Science, and…
On Dec. 16, 2025, researchers from the International Vaccines Access Center (IVAC) reported that as of mid-December, flu,…

On Dec. 15, 2025, the U.S. Department of Commerce announced that Montana State University will be home to…

On Dec. 12, 2025, the Texas Animal Health Commission (TAHC) and the United States Department of Agriculture’s (USDA)…
On Dec. 12, 2025, the Center for Infectious Disease Research & Policy (CIDRAP) reported COVID-19 rates in the…