Amneal Pharmaceuticals donated hydroxychloroquine sulfate to state of Texas
On Mar. 26, 2020, Amneal Pharma announced it had donated 1,000,000 hydroxychloroquine sulfate tablets to the Texas State…

On Mar. 26, 2020, Amneal Pharma announced it had donated 1,000,000 hydroxychloroquine sulfate tablets to the Texas State…

On Mar. 26, 2020, Henry Schein announced the availability of an antibody rapid blood test, known as Standard…

On Mar. 26, 2020, iBio announced that immunization studies for its SARS-CoV-2 Virus-Like Particle program (‘IBIO-200’) were proceeding…

On Mar. 26, 2020, Thermo Fisher Scientific announced it had received the CE mark in the European Union…

On Mar. 26, 2020, iBio announced that it had entered into a development agreement with AzarGen Biotechnologies. Signed…

On Mar. 25, 2020, NASA’s Jet Propulsion Laboratory (JPL) in Southern California announced that after receiving more than…
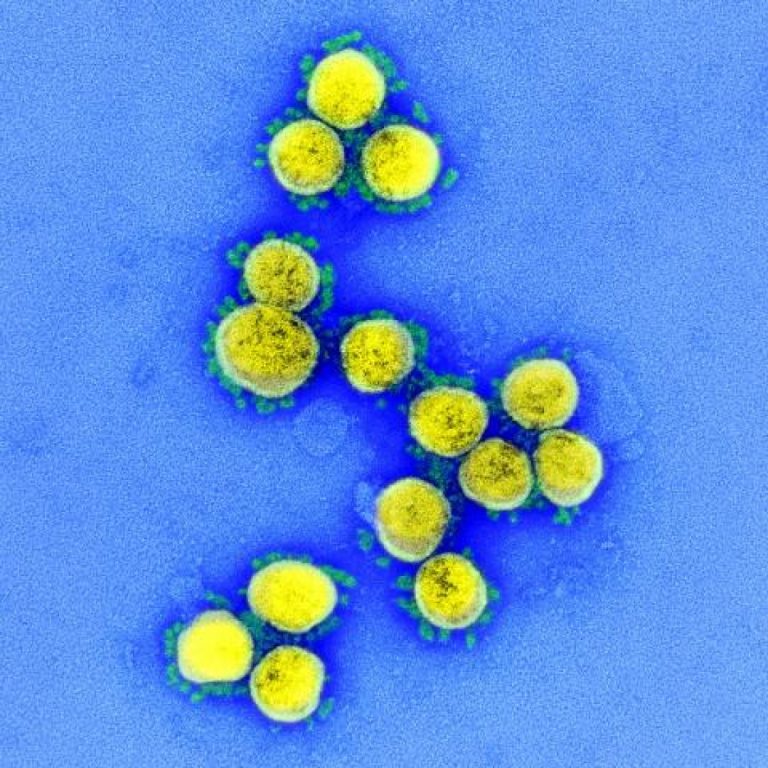
On Mar. 25, 2020, the National Library of Medicine (NLM), part of the National Institutes of Health, announced…

On Mar. 25, 2020, University of Oxford researchers announced they had launched the world’s first COVID-19 government response…

On Mar. 25, 2020, Philips announced that it was increasing the production of certain critical care products and…

On Mar. 25, 2020, a mobility and epidemiological study from a global consortium of researchers, led by the…

On Mar. 25, 2020, a consortium of life sciences companies announced an important collaboration to accelerate the development,…
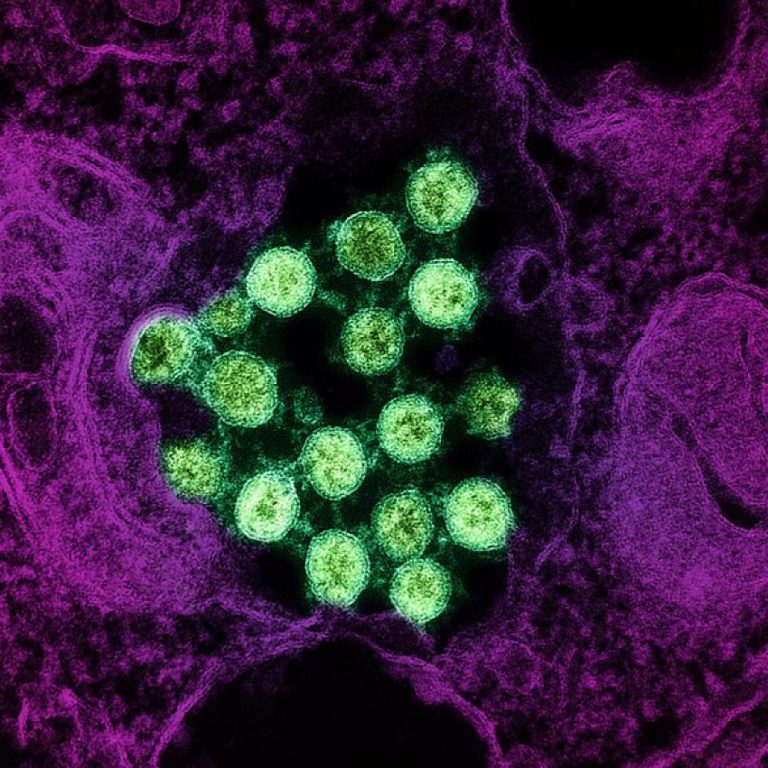
On Mar. 25, 2020, Mateon Therapeutics announced an update on its rapid antiviral response program targeting coronaviruses, initially…

On Mar. 25, 2020, Vir Biotech announced announced that it has identified multiple human monoclonal antibody (mAb) development…

On Mar. 25, 2020, Xencor announced a technology license agreement with Vir Biotech in which Vir will have…

On Mar. 25, 2020, the Jackson Laboratory (JAX) announced it was working to ensure that the worldwide research…

On Mar. 25, 2020, CHF Solutions announced the use of its Aquadex therapy in the treatment of patients…

On Mar. 25, 2020, Merck announced it had donated 300,000 masks to New Jersey’s Office of Homeland Security…

On Mar. 25, 2020, the Perelman School of Medicine at the University of Pennsylvania announced it had established…

On Mar. 24, 2020, Partner Therapeutics that Leukine (sargramostim, rhu-GM-CSF) is being assessed in the SARPAC trial (sargramostim…

On Mar. 24, 2020, the Andrew and Corey Morris-Singer Foundation donated $1.6 million to OHSU to support the…

On Mar. 24, 2020, T2 Biosystems announced it had entered into a worldwide licensing agreement for a rapid…

On Mar. 24, 2020, Windtree Therapeutics announced it will study its proprietary KL4 surfactant to potentially mitigate the…
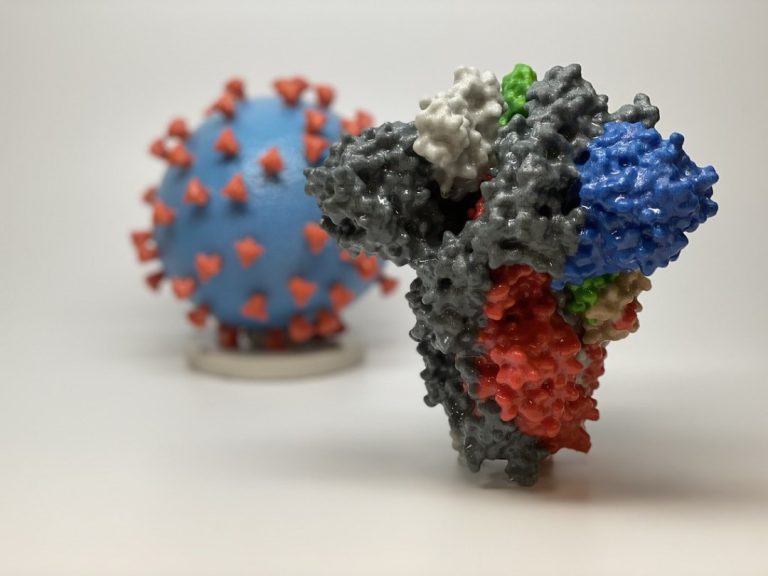
On Mar. 24, 2020, to help in educating the public and in the hope of better conveying the…
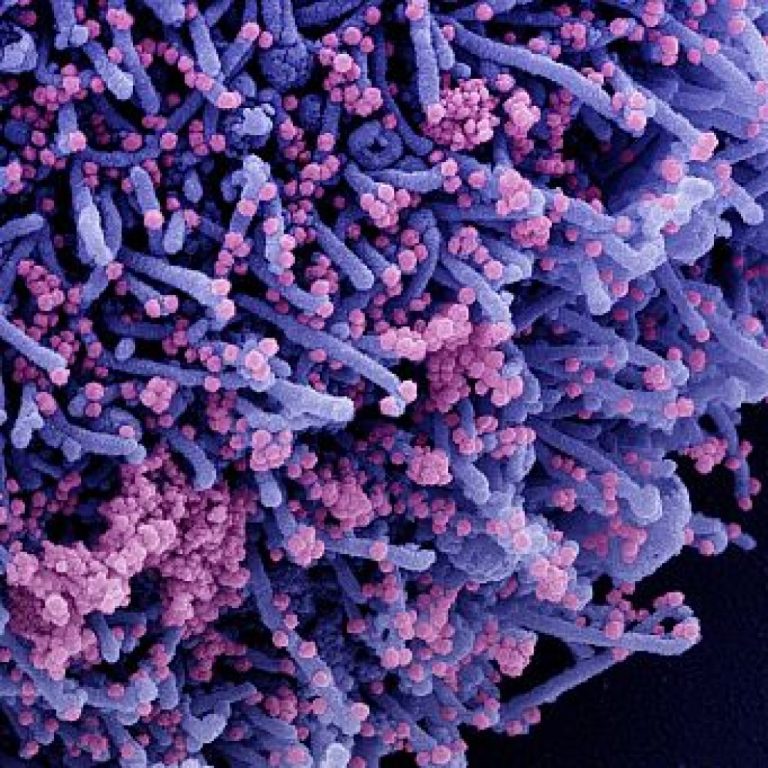
On Mar. 24, 2020, the University of Alberta (U of A) announced that a Public health study was…

On Mar. 24, 2020, Bausch Health announced it had donated medicines and health care products to assist in…

On Mar. 24, 2020, PerkinElmer announced the U.S. Food and Drug Administration (FDA) had provided Emergency Use Authorization…

On Mar. 24, 2020, a new international project aims to enroll 500 COVID-19 patients to search for genetic…

On Mar. 24, 2020, three Oxford University-based COVID-19 projects were among the first to benefit from a share…

On Mar. 24, 2020, bioMerieux announced that BioFire Diagnostics, its subsidiary specialized in syndromic infectious disease testing, had…
On Mar. 24, 2020, City of Hope announced that it had employed its vast expertise using the immune…