FDA issued Emergency Use Authorization for potential COVID-19 treatment
On May 1, 2020, the FDA issued an Emergency Use Authorization (EUA) for the investigational antiviral drug remdesivir…

On May 1, 2020, the FDA issued an Emergency Use Authorization (EUA) for the investigational antiviral drug remdesivir…
On May 1, 2020, a University of Southern California (USC) study suggested that temporarily suppressing the body’s immune…

On May 1, 2020, the CDC launched the National Healthcare Safety Network (NHSN) COVID-19 Module Data Dashboard showing…

On May 1, 2020, the U.S. Dept. of Veterans Affairs (VA) announced participation in a series of clinical…

On May 1, 2020, the CDC launched the SARS-CoV-2 Sequencing for Public Health Emergency Response, Epidemiology and Surveillance…

On May 1, 2020, Moderna and Lonza announced a 10-year strategic collaboration agreement to enable larger scale manufacture…

On Apr. 30, 2020, The California Institute for Regenerative Medicine (CIRM) awarded $750,000 to City of Hopeメs John…

On Apr. 30, 2020, Vaxart announced it has obtained positive pre-clinical results for its COVID-19 vaccine candidates, with…

On Apr. 30, 2020, Mateon Therapeutics reported several peer-reviewed publications derived from its R&D effort against COVID-19. Published…

On Apr. 30, 2020, Eiger BioPharmaceuticals announced that the first patients have been dosed in a Phase 2…
On Apr. 30, 2020, an international team of more than 120 scientists has detailed the impact of 75…
On Apr. 30, 2020, BioSig Technologies announced that an article titled, “The IMPDH inhibitor merimepodib provided in combination…
On Apr. 30, 2020, Emory University researchers are taking part in a multi-site study across the U.S. to…
On Apr. 30, 2020, Evotec announced that its Seattle-based subsidiary Just – Evotec Biologics hasd entered into a…

On Apr. 30, 2020, INOVIO announced an agreement to expand its manufacturing partnership with the German contract manufacturer…

On Apr. 30, 2020, the FDA included, under the ventilator emergency use authorization (EUA), a ventilator developed by…

On Apr. 30, 2020, Mesoblast announced a Phase 2/3 randomized, placebo-controlled trial to rigorously confirm whether its allogeneic…

On Apr. 30, 2020, AstraZeneca and the University of Oxford announced an agreement for the global development and…

On Apr. 30, 2020, University of Oxford announced an agreement with the UK-based global biopharmaceutical company AstraZeneca for…
On Apr. 30, 2020, FUJIFILM announced that it will reserve manufacturing capacity for a future COVID-19 therapy for…

On Apr. 30, 2020, the research community reacted with alarm and anger to the National Institutes of Health’s…
On Apr. 30, 2020, the University of Oxford announced an agreement with the UK-based global biopharmaceutical company AstraZeneca…

On Apr. 29, 2020, Chimerix announced initiation of a Phase 2/3 study of dociparstat sodium (DSTAT) in COVID-19…

On Apr. 29, 2020, BioReference Laboratories, an OPKO Health company, announced that it will continue to prioritize COVID-19…
On Apr. 29, 2020, researchers at Montefiore Health System and Albert Einstein College of Medicine and NYU Langone…

On Apr. 29, 2020, the NIH announced a new $1.5 billion federal stimulus funding initiative aimed to speed…
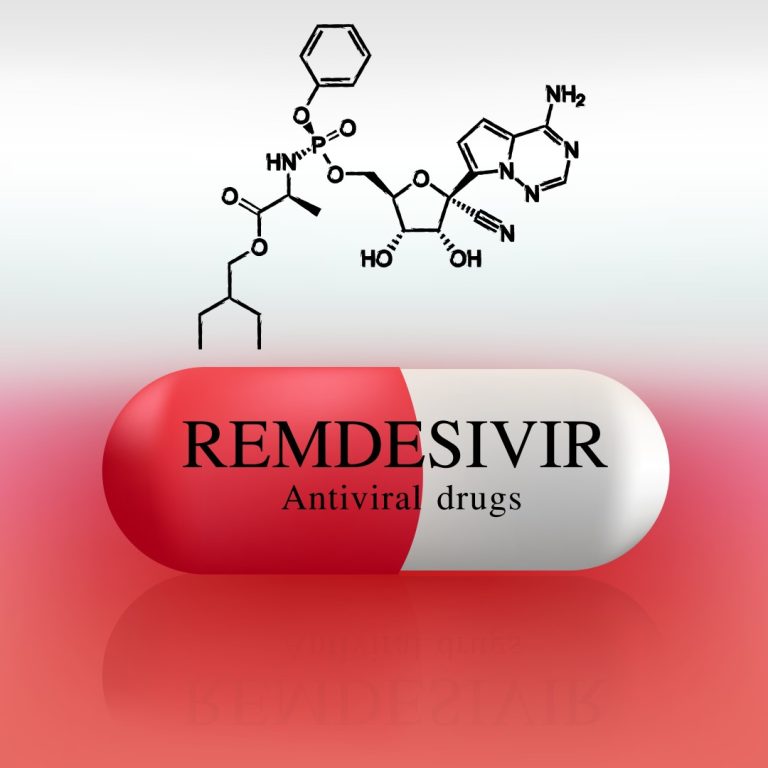
On Apr. 29, 2020, the National Institutes of Health (NIH) announced that hospitalized patients with advanced COVID-19 and…

On Apr. 29, 2020, researchers at Montefiore Health System and Albert Einstein College of Medicine announced that they…
On Apr. 29, 2020, a team of Texas A&M University researchers asked hundreds of frontline medical workers to…

On Apr. 29, 2020, the U.S. Dep. of Veterans Affairs (VA) announced a partnership with the XPRIZE Foundationメs…