Tuberculosis in the U.S. increased slightly during 2022 although it remained lower than prepandemic years
On Mar. 24, 2023, the U.S. Centers for Disease Control and Prevention (CDC) announced that Tuberculosis (TB) in…
On Mar. 24, 2023, the U.S. Centers for Disease Control and Prevention (CDC) announced that Tuberculosis (TB) in…

On Mar. 24. 2023, antibiotics do not reduce the risk of dying in adults hospitalized with COVID-19, the…

On Mar. 23. 2023, researchers at Washington University School of Medicine in St. Louis and the Veterans Affairs…
On Mar. 21, 2023, Quest Diagnostics announced that it had introduced two innovative Post-COVID-19 panels. These laboratory tests…

On Mar. 20, 2023, NIH scientists announced that the magnitude and quality of a key immune cellメs response…

On Mar. 16, 2023, Pfizer announced that the U.S. Food and Drug Administration’s Antimicrobial Drugs Advisory Committee (AMDAC)…
On Mar. 14, 2023, the U.S. Food and Drug Administration amended the emergency use authorization (EUA) of the…

On Mar. 13, 2023, Mayo Clinic published a study that showed the COVID-19 pandemic was linked to a…

On Mar. 8, 2023, QuidelOrtho announced that it had been granted a De Novo request from the U.S….

On Mar. 1, 2023, BioNTech and Pfizer announced that they had submitted an application to the U.S. Food…
On Feb. 24, 2023, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for…

On Feb. 24, 2023, Moderna announced will make certain contingent development, commercial and regulatory milestone payments to the…
On Feb. 21, 2023, Gilead Sciences announced positive data from three retrospective real-world studies which demonstrated that initiation…

On Feb. 20, 2023, results from a randomized clinical trial of Ivermectin in a higher-hose and longer duration…

On Feb. 17, 2023, Moderna announced that Health Canada had authorized the use of its Omicron-targeting bivalent COVID-19…
On Feb. 16, 2023, it was reported in the Lancet that protection from past infection against re-infection from…

On Feb. 15, 2023, Moderna announced it will continue to offer its COVID-19 vaccines for free, even after…

On Feb. 13, 2023, Novavax announced a modification to its existing agreement with the U.S. Department of Health…
On Feb. 8, 2023, BD announced that it had received Emergency Use Authorization from the U.S. Food and…

On Feb. 7, 2023, an NIH funded study by University of North Carolina reported that Bivalent booster vaccines…

On Jan. 26, 2023, Centers for Disease Control and Prevention (CDC) reported that U.S. household spread of flu…
On Jan. 25, 2023, researchers at Oregon Health & Science University (OHSU) reported that Immunity from COVID-19 appears…
On Jan. 25, 2023, Roche and its subsidiary TIB Molbiol announced they had developed a COVID-19 PCR test…

On Jan. 18, 2023, Novavax announced that partner SK bioscience had received expanded manufacturing and marketing approval from…
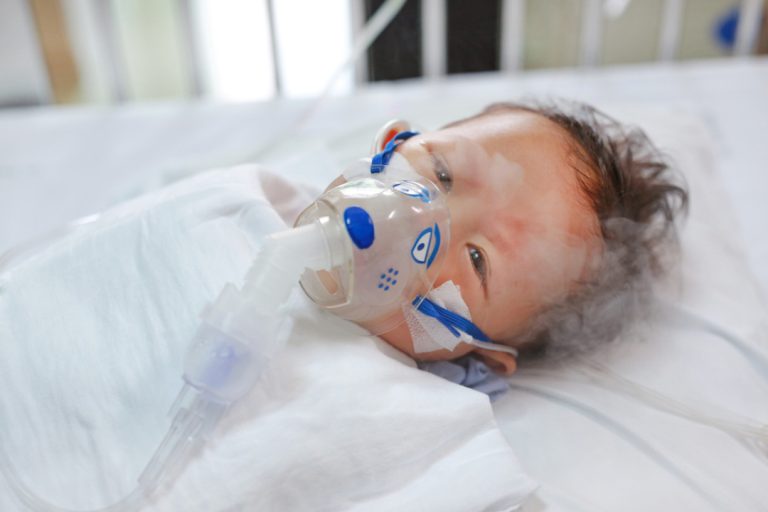
On Jan. 17, 2023, Moderna announced positive topline data from its ConquerRSV Phase 3 pivotal efficacy trial of…

On Jan. 11, 2023, the U.S. Food and Drug Administration reported that a single booster dose with an…
On Jan. 10, 2023, the U.S. National Institutes of Health (NIH) reported that antiviral treatments can help reduce…

On Jan. 5, 2023, the National Institutes of Health, in collaboration with the Administration for Strategic Preparedness and…

On Jan. 5, 2023, Novavax announced that Spain’s Public Health Commission, Italy’s Ministry of Health, and France’s Haute…

On Dec. 23, 2022, BioNTech and Fosun Pharmaceutical announced that they had received the certificates of registration as…