CDC launched モBridge Access Programヤ to provide free COVID-19 vaccines to uninsured and underinsured adults
On Jul. 13, 2023, the Centers for Disease Control and Prevention (CDC) announced the launch of the Bridge…

On Jul. 13, 2023, the Centers for Disease Control and Prevention (CDC) announced the launch of the Bridge…

On Jul. 6, 2023, University of Illinois Urbana-Champaign researchers reported they can detect exposure to the SARS-CoV-2 virus…

On Jun. 16, 2023, the U.S. Food and Drug Administration’s (FDA) Vaccines and Related Biological Products Advisory Committee…
On Jun. 6, 2023, the U.S. Food and Drug Administration (FDA) granted granted marketing authorization for the Cue COVID-19…
On May 31, 2023, the World Health Organization announced it had assigned simple, easy to say and remember…

On May 26, 2023, Novavax announced that Nuvaxovid (NVX-CoV2373) had been recommended for full Marketing Authorization (MA) for…

On May 25, 2023, the U.S. Food and Drug Administration (FDA) approved the oral antiviral Paxlovid (nirmatrelvir tablets…

On May 25, 2023, the National Institutes of Health announced Initial findings from a study of nearly 10,000…

On May 19, 2023, HealthPartners Institute researchers have published new data in JAMA Network Open that shows monovalent…
On May 11, 2023, a National Institutes of Health supported long-term study reported that among people who have…

On May 11, 2023, the U.S. Departments of Health and Human Services announced an end of the COVID-19…
On May 5, 2023, the World Health Organization (WHO) ended its declaration of COVID-19 as a global health…
On Apr. 27, 2023, a NIAID-led Phase 2 trial compared two Pfizer bivalent mRNA COVID-19 boosters found that…

On Apr. 24, 2023, the U.S. Food and Drug Administration (FDA) authorized Status COVID-19 Antigen Rapid Test for…

On Apr. 23, 2023, the most comprehensive state-by-state analysis of the impacts of COVID-19 across the USA, published…

On Apr. 14, 2023, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization for the…

On Apr. 10, 2023, the U.S. Department of Health and Human Services (HHS) announced it was spending over…

On Apr. 4, 2023, National Institutes of Health (NIH) funded research was announced from the University of Rochester…

On Mar. 30, 2023, Moderna and the Government of the Republic of Kenya announced they had finalized an…

On Mar. 24. 2023, antibiotics do not reduce the risk of dying in adults hospitalized with COVID-19, the…
On Mar. 24, 2023, the U.S. Centers for Disease Control and Prevention (CDC) announced that Tuberculosis (TB) in…

On Mar. 23. 2023, researchers at Washington University School of Medicine in St. Louis and the Veterans Affairs…
On Mar. 21, 2023, Quest Diagnostics announced that it had introduced two innovative Post-COVID-19 panels. These laboratory tests…

On Mar. 20, 2023, NIH scientists announced that the magnitude and quality of a key immune cellメs response…

On Mar. 16, 2023, Pfizer announced that the U.S. Food and Drug Administration’s Antimicrobial Drugs Advisory Committee (AMDAC)…

On Mar. 14, 2023, the U.S. Food and Drug Administration amended the emergency use authorization (EUA) of the…

On Mar. 13, 2023, Mayo Clinic published a study that showed the COVID-19 pandemic was linked to a…

On Mar. 8, 2023, QuidelOrtho announced that it had been granted a De Novo request from the U.S….

On Mar. 1, 2023, BioNTech and Pfizer announced that they had submitted an application to the U.S. Food…
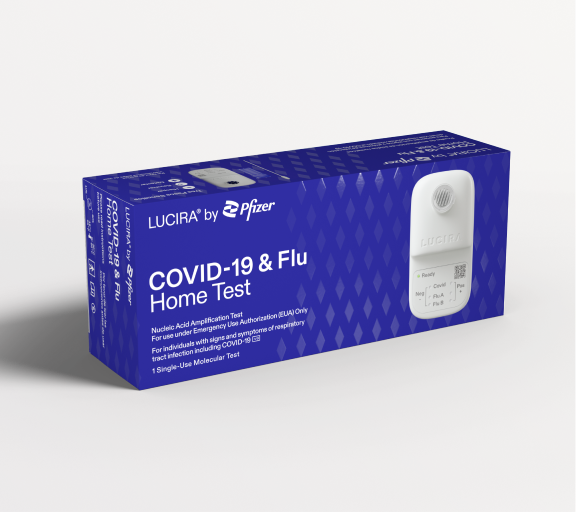
On Feb. 24, 2023, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for…