Hydroxychloroquine does not benefit adults hospitalized with COVID-19
On Nov. 9, 2020, a National Institutes of Health (NIH) clinical trial evaluating the safety and effectiveness of…
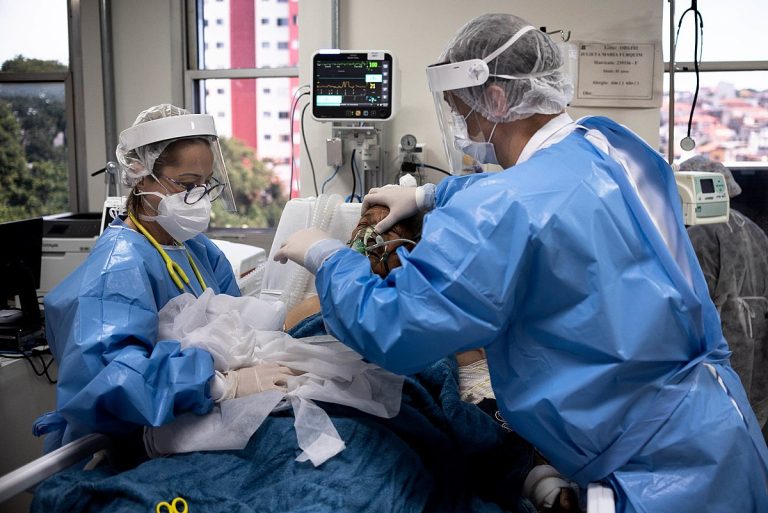
On Nov. 9, 2020, a National Institutes of Health (NIH) clinical trial evaluating the safety and effectiveness of…

On Nov. 9, 2020, a study published in JAMA reported the effect of hydroxychloroquine on clinical status at…

On Nov. 9, 2020, the FDA granted Emergency Use Authorization (EUA) for Eli Lilly’s investigational neutralizing antibody bamlanivimab…
On Nov. 9, 2020, Pfizer and BioNTech announced their mRNA-based vaccine candidate, BNT162b2, against SARS-CoV-2 had demonstrated evidence…

On Nov. 9, 2020, Corvus Pharmaceuticals announced that it had completed patient enrollment in its Phase 1 study…
On Nov. 9, 2020, the Fred Hutchinson Cancer Research Center announced the start of volunteer enrollment for a…

On Nov. 8, 2020, ImmunityBio announced positive study results for their human Ad5 (hAd5) COVID-19 vaccine candidate, which…

On Nov. 8, 2020, research from the U.S. had shown that white-tailed deer were being infected with SARS-CoV-2,…
On Nov. 6, 2020, Mesa Biotech announced it had been awarded a contract up to $13 million from…

On Nov. 6, 2020, Humanigen announced that it had entered into a Cooperative Research and Development Agreement (CRADA)…

On Nov. 5, 2020, Neoleukin Therapeutics announced the publication in Science of research describing novel molecules designed to…

On Nov. 5, 2020, Novartis announced data from an interim analysis for the randomized, double-blind, placebo-controlled CAN-COVID trial…

On Nov. 5, 2020, Denmark announced strict lockdown rules in the north of the country after authorities discovered…

On Nov. 5, 2020, RELIEF THERAPEUTICS and NeuroRx announced that the independent Data Monitoring Committee (DMC) voted unanimously…

On Nov. 5, 2020, Regeneron announced an update from the independent data monitoring committee (DMC) for the UK-based…

On Nov. 4, 2020, the University of Oxford announced that its rapid COVID-19 test was being used at…

On Nov. 4, 2020, Mateon Therapeutics announced the receipt of approval from Instituto Nacional de Salud (INS), the…
On Nov. 4, 2020, Humanigen announced positive interim Phase 3 data of lenzilumab in patients hospitalized with COVID-19….

On Nov. 4, 2020, Novavax announced the signing of a non-binding Heads of Terms document with the Australian…
On Nov. 4, 2020, using the cutting-edge genetic editing technique known as CRISP ‘lab on a chip’ technology,…

On Nov. 3, 2020, Medigen Vaccine Biologics (MVC) and the National Institute of Hygiene and Epidemiology (NIHE), a…

On Nov. 3, 2020, Humanigen announced the execution of its first licensing transaction in the Asia-Pacific Region with…

On Nov. 3, 2020, OraSure Technologies announced its DNA Genotek subsidiary has received Emergency Use Authorization from the…

On Nov. 2, 2020, Innovation Pharma announced receipt of written feedback from the FDA that is in general…

On Nov. 2, 2020, Immunic announced that the company had enrolled and randomized 200 patients, pre-specified in the…

On Nov. 2, 2020, Pacific Northwest National Laboratory (PNNL) announced that in the Spring of 2020, as news…

On Nov. 2, 2020, Novavax announced the expansion of its Maryland campus to accommodate the company’s rapid growth…

On Nov. 2, 2020, ACON Laboratories announced the launch of its Flowflexル SARS-COV-2 Antigen Rapid Test. The Flowflex…
On Nov. 1, 2020, scientists announced that increasing evidence shows that both natural and synthetic antimicrobial peptides (AMPs),…

On Nov. 1, 2020, the U.S. Department of Defense announced the start of rapid, on-site COVID-19 testing for…