USAID announced Initiative for Global Vaccine Access to accelerate vaccine access and delivery around the world
On Dec. 6, 2021, the USAID announced the foundation of a new whole-of-government effort, the Initiative for Global…

On Dec. 6, 2021, the USAID announced the foundation of a new whole-of-government effort, the Initiative for Global…

On Dec. 5, 2021, Sorrento Therapeutics announced the peer-reviewed publication of a series of novel SARS-CoV-2 MPro inhibitors…

On Dec. 5, 2021, Roche announced that its planned to launch the SARS-CoV-2 & Flu A/B Rapid Antigen…

On Dec. 5, 2021, Johnson & Johnson announced preliminary results from an independent study, including a subset of…

On Dec. 3, 2021, a report from the U.S. Department of Health and Human Services (HHS) found that…
On Dec. 1, 2021, T2 Biosystems announced that its T2SARS-CoV-2 Panel detected the Omicron COVID-19 variant (B.1.1.529). The…

On Dec. 1, 2021, Fulgent Genetics confirmed that the Companyメs RT-PCR test for SARS-CoV-2, the virus that causes…

On Dec. 1, 2021, Moderna announced a revised supply agreement with the UK government for up to 60…
On Dec. 1, 2021, the California and San Francisco Departments of Public Health confirmed that a recent case…
On Nov. 30, 2021, Inovio Pharma announced the company was rapidly moving to evaluate its COVID-19 DNA vaccine…
On Nov. 30, 2021, LabCorp announced that it had begun offering observed self-collection for COVID-19 PCR testing in…

On Nov. 29, 2021, Thermo Fisher Scientific confirmed that its polymerase chain reaction (PCR) TaqPath COVID-19 Combo Kit…
On Nov. 29, 2021, Hologic announced that its three SARS-CoV-2 tests all detect the recently emerged Omicron variant of…
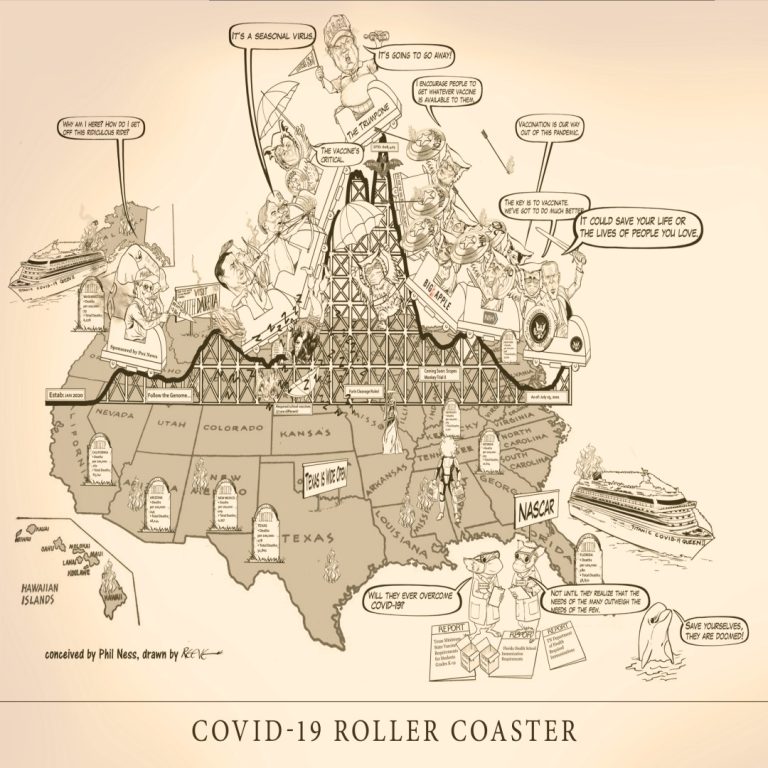
The COVID-19 Roller Coaster is a wild ride, strap yourself in and hold on… Cast of Characters: Senator…

On Nov. 26, 2021, Merck provided an update on the MOVe-OUT study of molnupiravir, an investigational oral antiviral…

On Nov. 25, 2021, Pfizer and BioNTech announced that the Committee for Medicinal Products for Human Use (CHMP)…

On Nov. 24, 2021, Novavax announced its submission to the Singapore Health Sciences Authority for interim authorization of…

On Nov. 24, 2021, Johnson & Johnson announced the U.S. Food and Drug Administration (FDA) had issued Emergency…

On Nov. 23, 2021, the World Health Organization’s COVID-19 Technology Access Pool and the Medicines Patent Pool finalized…
On Nov. 22, 2021, Tonix Pharmaceuticals announced the publication of ‘Sangivamycin is highly effective against SARS-CoV-2 in vitro…
On Nov. 22, 2021, Pfizer announced topline results from a longer-term analysis of the safety and efficacy of…

On Nov. 22, 2021, the U.S. Food and Drug Administration (FDA) authorized another over-the-counter (OTC) COVID-19 antigen test. The…

On Nov. 19, 2021, the U.S. Food and Drug Administration (FDA) announced that it had extended the emergency…

On Nov. 19, 2021, Pfizer and BioNTech announced that the U.S. Food and Drug Administration (FDA) had expanded…
On Nov. 18, 2021, Pfizer announced an agreement with the U.S. government to supply 10 million treatment courses…

On Nov. 17, 2021, Zosano Pharma announced that the Philippine Food and Drug Administration had granted emergency use…

On Nov. 17, 2021, Novavax and and Serum Institute of India announced that the Philippine Food and Drug…

On Nov. 17, 2021, GlaxoSmithKline and Vir Biotechnology announced U.S. government contracts totalling approximately $1 billion (USD) to…

On Nov. 17, 2021, Novavax announced that the European Medicines Agency (EMA) had begun its evaluation of an…

On Nov. 16, 2021, an agreement was announced that enabled the European Union and European Economic Area countries…