California Department of Public Health Reported 13 Confirmed Human Cases of Bird Flu
On Oct. 18, 2024, the California Department of Public Health (CDPH) reported that a total of 13 human…

On Oct. 18, 2024, the California Department of Public Health (CDPH) reported that a total of 13 human…
On Oct. 10, 2024, health officials from the U.S. Centers for Disease Control and Prevention announced they had…
On Oct. 10, 2024, the U.S. Centers for Disease Control and Prevention announced that a 2023 presumed outbreak…

On Sep. 6, 2024, the Centers for Disease Control and Prevention (CDC) confirmed a human case of avian…

On Aug. 23, 2024, the U.S. Centers for Disease Control and Prevention (CDC) reported that two human infections…

On Aug. 16, 2024, the U.S. Centers for Disease Control and Prevention (CDC) reported that COVID-19 infections are…

On Jul. 26, 2024, the Maryland Department of Health advised consumers not to eat certain deli meats produced…

On Jul. 3, 2024, the Centers for Disease Control and Prevention (CDC) reported that a human case of…

On Jun. 27, 2024, the U.S. Centers for Disease Control and Prevention’s (CDC) recommended the updated 2024-2025 COVID-19…

On Jun. 26, 2024, the U.S. Centers for Disease Control and Prevention (CDC) updated its recommendation for the…

On Jun. 25, 2024 the Centers for Disease Control and Prevention (CDC) issued a Health Alert Network (HAN)…

On Jun. 14, 2024 the Centers for Disease Control and Prevention (CDC) reported data from this study suggested…

On Jun. 4, 2024, the Minnesota Department of Health reported that Avian Influenza A(H5N1) was detected in dairy…

On May 30, 2024, the U.S. Centers for Disease Control and Prevention (CDC) announced that a second human…

On Apr. 12, 2024, the U.S. Centers for Disease Control and Prevention (CDC) announced that Vietnam had reported…

On Apr. 5, 2024, the U.S. Centers for Disease Control and Prevention (CDC) announced that Texas had reported…

On Mar. 29, 2024, the U.S. Centers for Disease Control and Prevention (CDC) reported the first U.S. human…

On Mar. 8, 2024, the U.S. Food and Drug Administration’s (FDA) Vaccines and Related Biological Products Advisory Committee…

On Feb. 29, 2024, Valneva announced that the U.S. Centers for Disease Control and Preventionメs (CDC) Advisory Committee…

On Feb. 28, 2024, the U.S. Centers for Disease Control and Preventionメs (CDC) Advisory Committee on Immunization Practices…

On Feb. 28, 2024, the CDC Advisory Committee on Immunization Practices’ (ACIP) announced a recommendation for adults ages…
On Feb. 16, 2024, the U.S. Centers for Disease Control and Prevention announced that ten people infected with…

On Feb. 12, 2024, the U.S. Centers for Disease Control and Prevention (CDC) announced that Cambodia had reported…

On Feb. 5, 2024, a U.S. Centers for Disease Control and Prevention (CDC) celebrated the opening of the…
On Dec. 22, 2023, the U.S. Centers for Disease Control and Prevention (CDC) projected the JN.1 variant of…
On Dec. 22, 2023, the U.S. Centers for Disease Control and Prevention (CDC) announced study results that showed…

On Dec. 18, 2023, the U.S. Centers for Disease Control and Prevention (CDC) announced a study that showed…
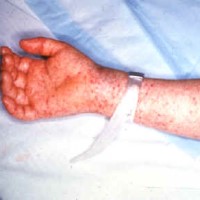
On Dec. 11, 2023, the U.S. Centers for Disease Control and Prevention (CDC) issued a Health Alert Network…

On Nov. 29, 2023, the BC Centre for Disease Controlr Disease Control (BCCDC) announced that a A passenger…

On Nov. 27, 2023, the CDCメs Tuberculosis Trials Consortium (TBTC) announced that it had launched an international clinical…