Moderna filed to expand the conditional Marketing Authorization for COVID-19 vaccine in the EU to include children ages 6-11 years
On Nov. 9, 2021, Moderna announced that it has submitted for a variation to the conditional marketing authorization…

On Nov. 9, 2021, Moderna announced that it has submitted for a variation to the conditional marketing authorization…

On Nov. 9, 2021, Inovio Pharma announced that the U.S. Food and Drug Administration (FDA) provided authorization to…

On Nov. 8, 2021, Regeneron announced that the European Commission had approved the casirivimab and imdevimab antibody cocktail,…

On Nov. 8, 2021, Cocrystal Pharma announced that its SARS-CoV-2 main protease inhibitors showed potent in vitro pan-viral…

On Nov. 5, 2021, the USDA’s (USDA) National Veterinary Services Laboratories announced confirmation of SARS-CoV-2 in two spotted…

On Nov. 5, 2021, Pfizer announced it was investigational novel COVID-19 oral antiviral candidate, PAXLOVID, significantly reduced hospitalization…

On Nov. 5, 2021, Chugai Pharmaceutical, announced that it had obtained approval from the Ministry of Health, Labour…

On Nov. 3, 2021, Novavax announced the company had filed for provisional approval of the vaccine to the…

On Nov. 3, 2021, Inovio Pharma announced that it had received authorization from India’s Central Drugs Standard Control…

On Nov. 1, 2021, Novavax announced the completion of its rolling submission to Health Canada for authorization of…

On Nov. 1, 2021, Novavax and Serum Institute of India announced that the National Agency of Drug and…

On Oct. 31, 2021, Moderna announced that the U.S. Food and Drug Administration (FDA) had notified the Company…
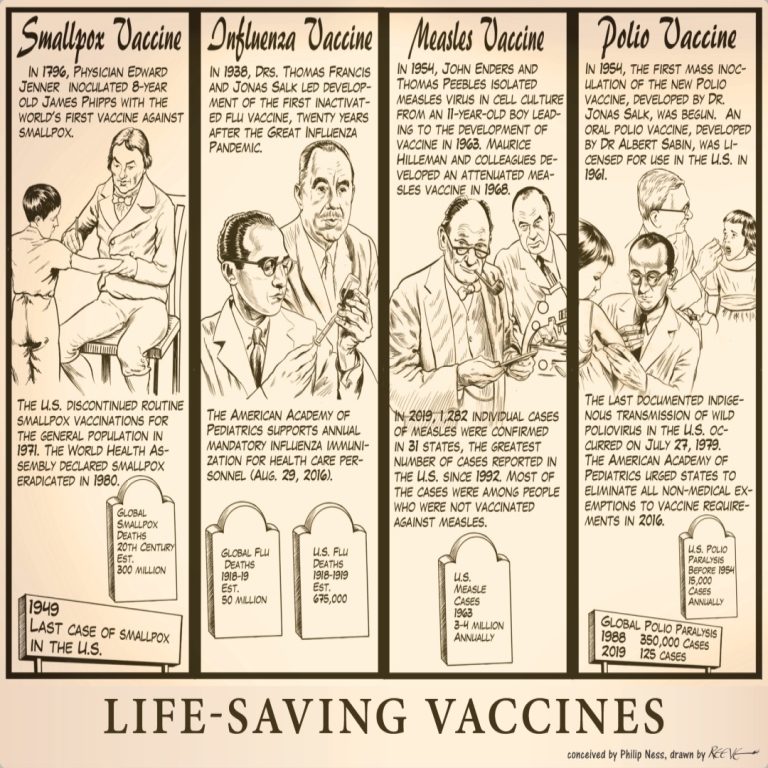
Our Life-Saving Vaccines cartoon illustrates four invaluable vaccines that have affected human civilization throughout history from smallpox and…

On Oct. 29, 2021, Oregon Health & Science University’s (OHSU) board of directors approved a project to expand…

On Oct. 29, 2021, Novavax announced the completion of its rolling submission to the Therapeutic Goods Administration (TGA)…
On Oct. 29, 2021, research led by scientists at the National Institutes of Health announced they had identified…

On Oct. 28, 2021, Pfizer and BioNTech announced that the U.S. government had purchased 50 million more doses…

On Oct. 27, 2021, researchers published interim results in The New England Journal of Medicine from a Phase…
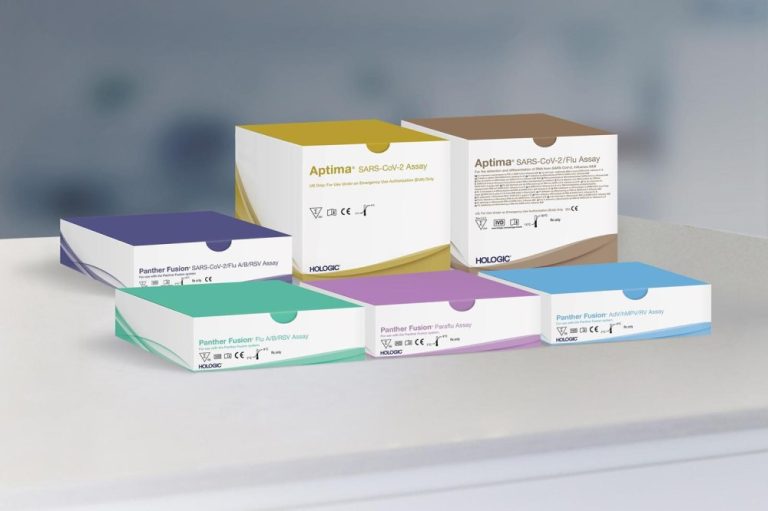
On Oct. 27, 2021, Hologic announced that its Aptima SARS-CoV-2/Flu Assay was available for the simultaneous detection and…

On Oct. 27, 2021, Novavax announced the completion of its rolling regulatory submission to the U.K. Medicines and…

On Oct. 26, 2021, BioNTech announce that the Company planned to initiate the construction of the first state-of-the-art…

On Oct. 26, 2021, Anixa Biosciences announced that, in conjunction with its partner, Cleveland Clinic, it had commenced…

On Oct. 26, 2021, Moderna announced a new Memorandum of Understanding (MoU) to make up to 110 million…

On Oct. 26, 2021, Moderna announced that Swissmedic had authorized a booster dose of Spikevax, the Company’s vaccine…

On Oct. 25, 2021, Moderna announced positive interim data from the Phase 2/3 study, called the KidCOVE study,…

On Oct. 25, 2021, Moderna announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human…

On Oct. 22, 2021, the U.S. Department of Veterans Affairs (VA) announced that it had begun offering Moderna…

On Oct. 22, 2021, the U.S. Centers for Disease Control and Prevention (CDC) reported two new U.S. human…

On Oct. 21, 2021, Pfizer and BioNTech announced topline results from a Phase 3 randomized, controlled trial evaluating…

On Oct. 21, 2021, Inovio Pharma announced the signing of a non-binding memorandum of understanding with Colombia’s Ministry…