NIH established initiative to systematically investigate and establish function of every human gene
On Sept. 27, 2022, the National Institutes of Health (NIH) announced it had launched a program to better…
On Sept. 27, 2022, the National Institutes of Health (NIH) announced it had launched a program to better…

On Sept. 28, 2022, Pfizer and BioNTech announced they have completed a submission to the U.S. Food and…

On Supt. 22, 2022, Pfizer announced an agreement to supply up to six million treatment courses of its…
On Sept. 20, 2022, The health authorities in Uganda declared an outbreak of Ebola after a case of…

On Sept. 16, 2022, Novavax announced that the Taiwan Food and Drug Administration had granted expanded emergency use…

On Sept. 16, 2022, Novavax announced that the Israel Ministry of Health had granted an import and use…

On Sept. 15, 2022, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) confirmed…
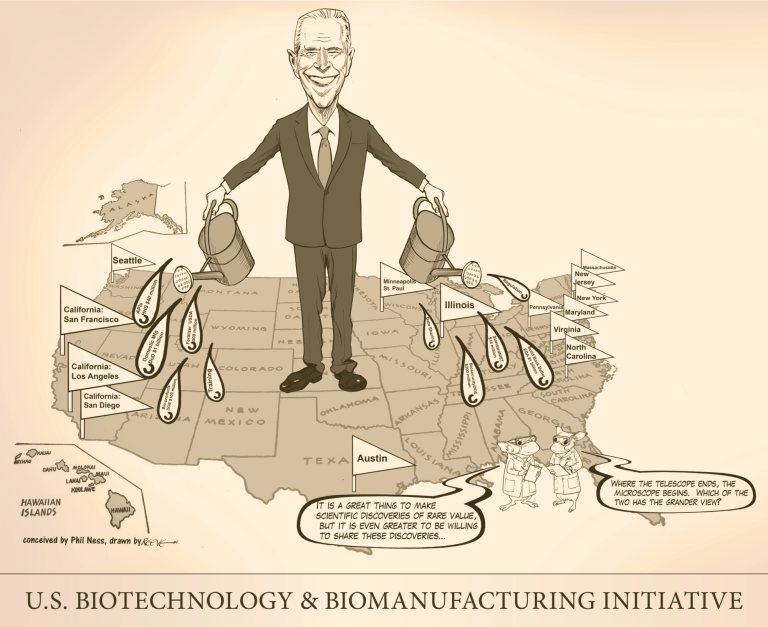
On Sept. 14, 2022, The U.S. Biotech Initiative was announced detailing new investments and resources to advance the…
On Sept. 13, 2022, Novavax and Serum Institute of India, the world’s largest vaccine manufacturer by volume, announced…

On Sept. 8, 2022, the Fred Hutchinson Cancer Center (FHCRC) announced that Stuart and Molly Sloan had pledged…
On Sept. 8, 2022, a clinical trial evaluating alternative strategies for administering the JYNNEOS mpox vaccine to increase…

On Sept. 7, 2022, the U.S. Food and Drug Administration (FDA) announced it had approved Revance Therapeutics’ DAXXIFY…

On Sept. 6, 2022, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service had reviewed…

On Sept. 2, 2022, Novavax announced that the World Health Organization (WHO) had approved a variation to allow…

On Sept. 2, 2022, Novavax announced that Swissmedic, the Swiss Agency for Therapeutic Products, had expanded its temporary…

On Sept. 1, 2022, Novavax announced that Nuvaxovid (NVX-CoV2373) COVID-19 vaccine had been recommended for expanded conditional marketing…

On Aug. 31, 2022, a new front-door to the University of Victoria opened to advance health and life…

On Aug. 31, 2022, the Yale New Haven Hospital broke ground on the $838 million, 505,000 square foot…

On Aug. 29, 2022, the U.S. Department of Health and Human Services (HHS) announced it had provided approximately…

On Aug. 26, 2022, Novavax announced that the Medicines and Healthcare products Regulatory Agency (MHRA) in the United…

On Aug. 26, 2022, Pfizer and BioNTech announced they had completed a submission to the European Medicines Agency…

On Aug. 22, 2022, the Food and Drug Administration (FDA) amended the emergency use authorization (EUA) of Moderna…

On Aug. 23, 2022, Moderna announced that the Government of Canada had exercised its option to purchase an…

On Aug. 22, 2022, Pfizer and BioNTech announced they had completed a submission to the U.S. Food and…
On Aug. 19, 2022, Novavax announced that the Novavax COVID-19 Vaccine, Adjuvanted (NVX-CoV2373) had received expanded emergency use…

On Aug. 18, 2022, Novavax announced that New Zealand’s Medsafe had granted expanded provisional approval for Nuvaxovid (NVX-CoV2373)…

On Aug. 15, 2022, Moderna announced that the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK…

On Aug. 12, 2022, Novavax announced that partner, SK bioscience, had received a Post Approval Change Application approval…

On Aug. 11, 2022, Roche announced that the U.S. Food and Drug Administration (FDA) had approved a supplemental…

On Aug. 9, 2022, Moderna announced an amendment to its agreement with the European Commission (EC) to convert…