Experimental NIH Sudan virus vaccine protected Macaques
On Feb. 2, 2023, a National Institutes of Health research group with extensive experience studying ebolavirus countermeasures successfully…
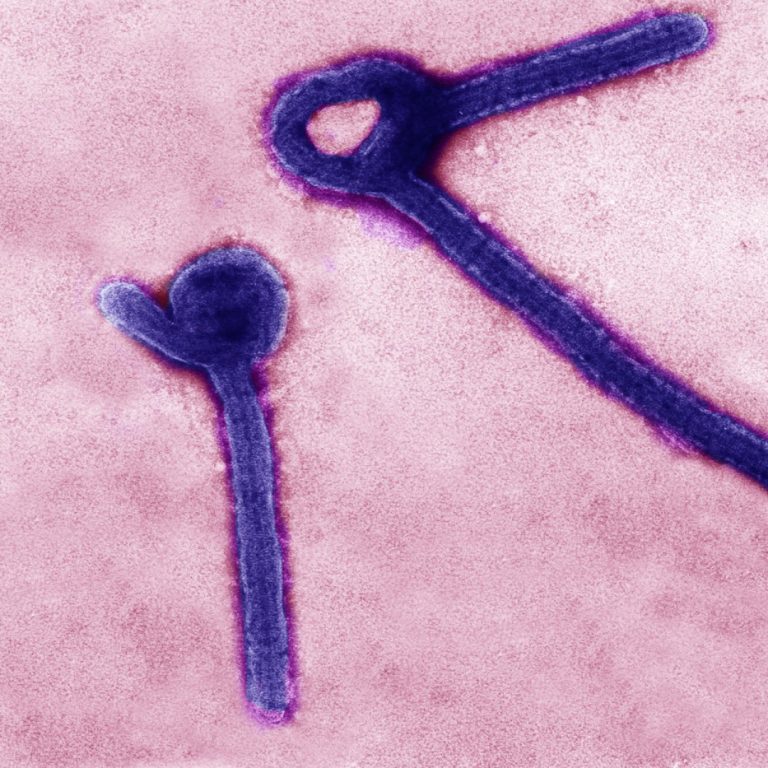
On Feb. 2, 2023, a National Institutes of Health research group with extensive experience studying ebolavirus countermeasures successfully…

On Jan. 31, 2023, a team of researchers led by the Innovation Center for Neurological Disorders and Department…

On Jan. 26, 2023, Twist Bioscience announced that it had begun offering high-quality synthetic DNA using its silicon…

On Jan. 26, 2023, Centers for Disease Control and Prevention (CDC) reported that U.S. household spread of flu…
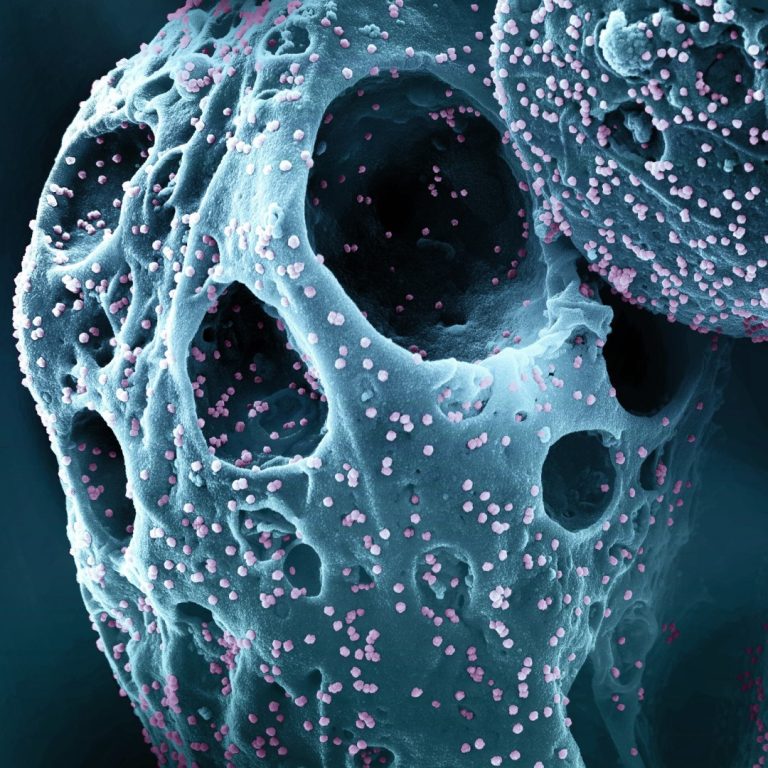
On Jan. 25, 2023, Roche and its subsidiary TIB Molbiol announced they had developed a COVID-19 PCR test…

Our Milestones in CRISPR cartoon illustrates several significant achievements in the development of the technology with commentary by…

On Jan. 19, 2023, researchers at ETH Zurich and the Swiss Federal research institute WSL announced they had…

On Jan. 18, 2023, Novavax announced that partner SK bioscience had received expanded manufacturing and marketing approval from…

On Jan. 18, 2023, the National Institutes of Health (NIH) announced that an investigational HIV vaccine regimen tested…

On Jan. 17, 2023, Moderna announced positive topline data from its ConquerRSV Phase 3 pivotal efficacy trial of…

On Jan. 17, 2023, the Montana Fish and Wildlife Commission (FWP) reported that juvenile grizzly bears tested positive…
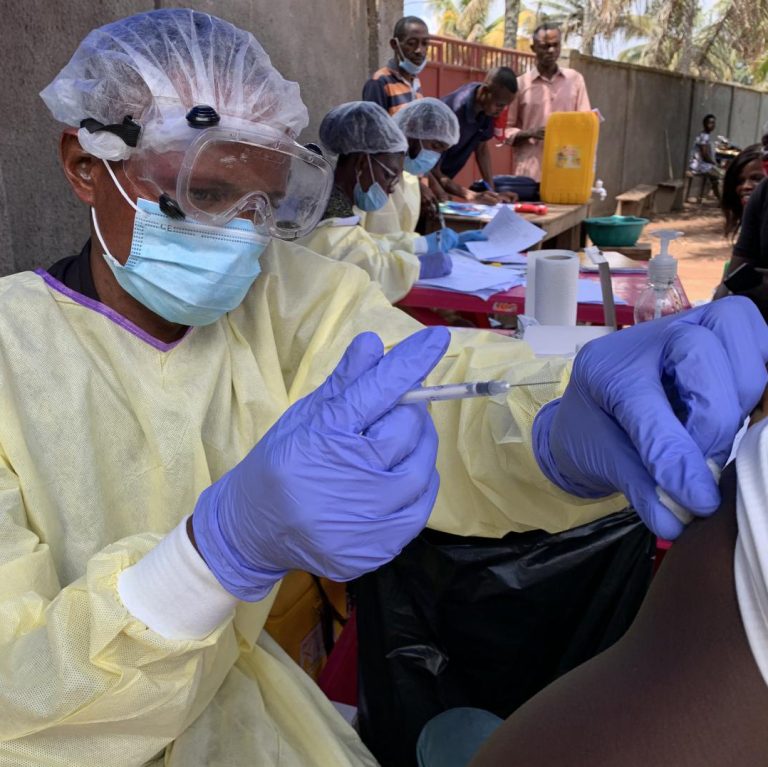
On Jan. 11, 2023, Uganda declared the end of the Ebola disease outbreak caused by Sudan ebolavirus, less…

On Jan. 11, 2023, Roche announced treatment of uncomplicated influenza and for post-exposure prophylaxis of influenza. Post-exposure prophylaxis…

On Jan. 10, 2023, the U.S. National Institutes of Health (NIH) reported that antiviral treatments can help reduce…

On Jan. 6, 2023, the Purdue Center for Cancer Research announced it was changing its name to the…
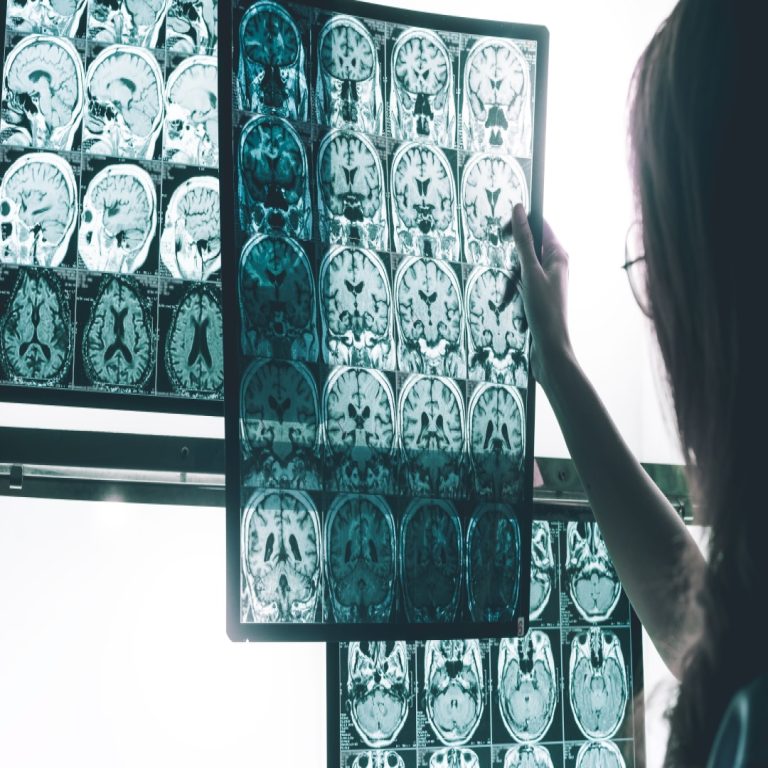
On Jan. 6, 2023, the U.S. Food and Drug Administration (FDA) approved Leqembi (lecanemab-irmb) via the Accelerated Approval…

On Jan. 5, 2023, Novavax announced that Spain’s Public Health Commission, Italy’s Ministry of Health, and France’s Haute…
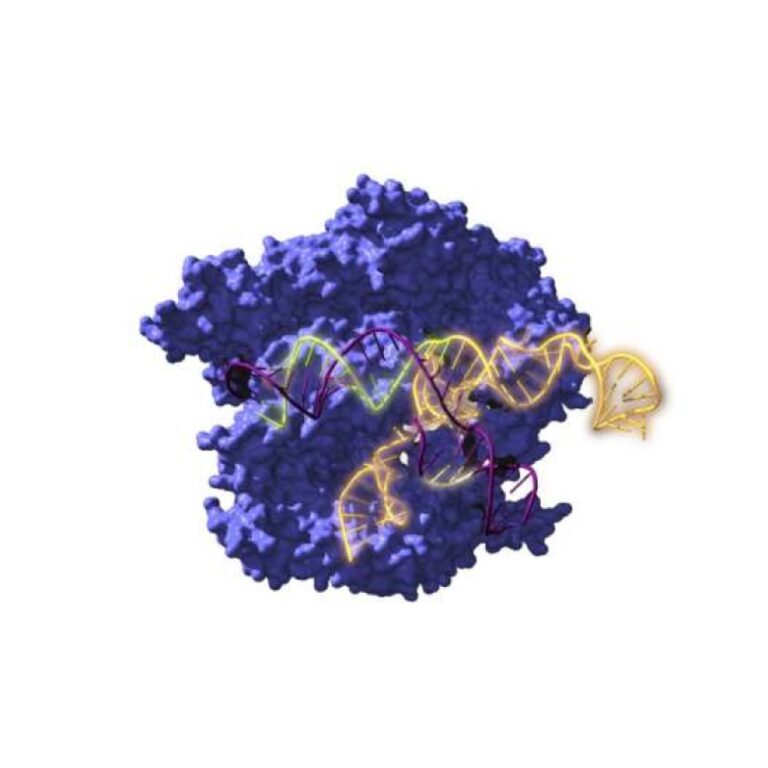
On Jan. 4, 2023, an international research group announced it had for the first time reconstructed ancestors dating…

On Jan. 1, 2023, a study published in the Lancet reported that concentrations of antibiotic residues found in…

Milestones in Polio illustrates the slow but steady advancements from its clinical description and discovery to widespread infection…

On Dec. 24, 2022, the U.S. Food and Drug Administration (FDA) approved updated labeling for Genentech’s capecitabine tablets…
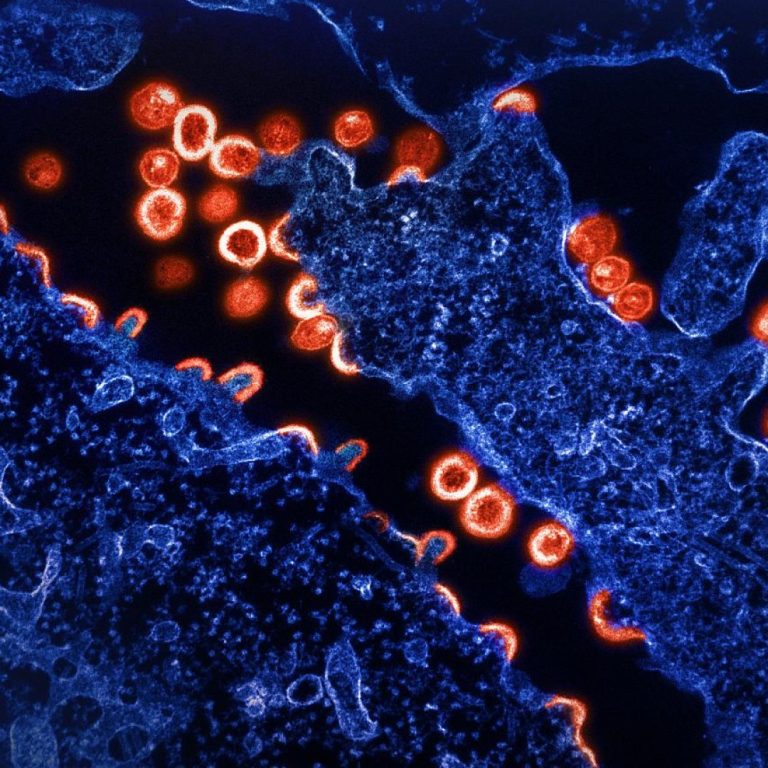
On Dec. 22, 2022, Gilead Sciences announced that Sunlenca (lenacapavir), in combination with other antiretroviral(s) (ARV), had been…

On Dec. 21, 2022, Moderna announced the the finalization of a strategic partnership with the United Kingdom (UK)…

On Nov. 12, 2022, the World Health Organization (WHO) issued a Disease Outbreak News (DON) on the circulating…
On Dec. 16, 2022, Novavax announced that the Standing Committee on Vaccination (STIKO) in Germany had expanded its…

On Dec. 16, 2022, Moderna announced that the European Medicines Agency’s Committee for Medicinal Products for Human Use…
On Dec. 15, 2022, the University of Oxford’s Ebola vaccine candidate has been shipped to Uganda, just 80…

On Dec. 13, 2022, Texas Biomed announced that it had been designated as a prime contractor by the…

On Dec. 11, 2022, Novavax announced that the Switzerland’s Federal Office of Public Health and Francees Haute Autorite…

On Dec. 10, 2022, CSL announced data affirming the long-term durability and safety of single-infusion HEMGENIX® (etranacogene dezaparvovec-drlb)…