Advisory Committee on Immunization Practices recommended universal Hepatitis B vaccination in adults aged 19-59 years
On Mar. 10, 2023, the Centers for Disease Control and Prevention (CDC) recommended the universal hepatitis B (HepB)…
On Mar. 10, 2023, the Centers for Disease Control and Prevention (CDC) recommended the universal hepatitis B (HepB)…

On Mar. 10, 2023, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved ZAVZPRET (zavegepant),…

On Mar. 9, 2023, the U.S. Food and Drug Administration (FDA) announced that it had published updates to…
On Mar. 8, 2023, Amphastar Pharmaceuticals announced that the U.S. Food and Drug Administration (FDA) had granted approval…

On Mar. 8, 2023, QuidelOrtho announced that it had been granted a De Novo request from the U.S….

On Mar. 7, 2023, the the World Organisation for Animal Health (WOAH) announced that France had reported an…

On Mar. 7, 2023, Abbott announced that it had received U.S. Food and Drug Administration clearance for what…
On Mar. 7, 2023, researchers at the National Institutes of Health reported the benefits of screening adult patients…

On Mar. 6, 2023, Merck announced that the U.S. Food and Drug Administration (FDA) had approved the addition…

On Mar. 1, 2023, BioNTech and Pfizer announced that they had submitted an application to the U.S. Food…
On Feb. 28, 2023, Public Health Delta & Menominee Counties (PHDM) was alerted to three cases of atypical…

On Feb. 28, 2023, a team from the University of Sydney reported that a flu virus found in…

On Feb. 26, 2023, the Cambodia International Health Regulations (IHR) National Focal Point (NFP) reported one confirmed case…
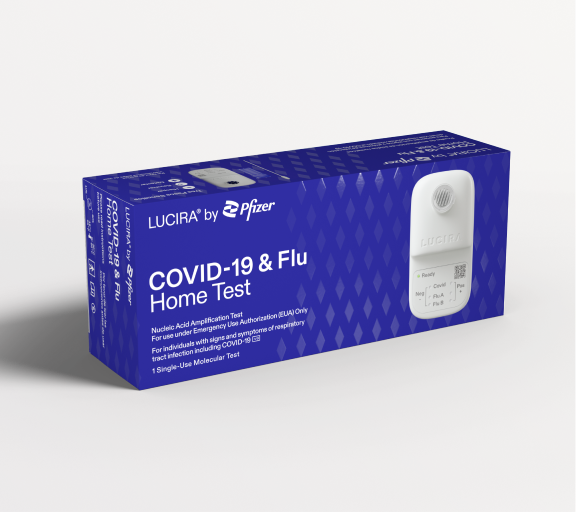
On Feb. 24, 2023, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for…

On Feb. 24, 2023, Moderna announced will make certain contingent development, commercial and regulatory milestone payments to the…

On Feb. 22, 2023, the Centers for Disease Control and Prevention (CDC) reported that the 2022-2023 flu vaccines…

On Feb. 20, 2023, results from a randomized clinical trial of Ivermectin in a higher-hose and longer duration…

On Feb. 17, 2023, Moderna announced that Health Canada had authorized the use of its Omicron-targeting bivalent COVID-19…

On Feb. 16, 2023, the Government of China announced it will plant less than 1% of its corn…
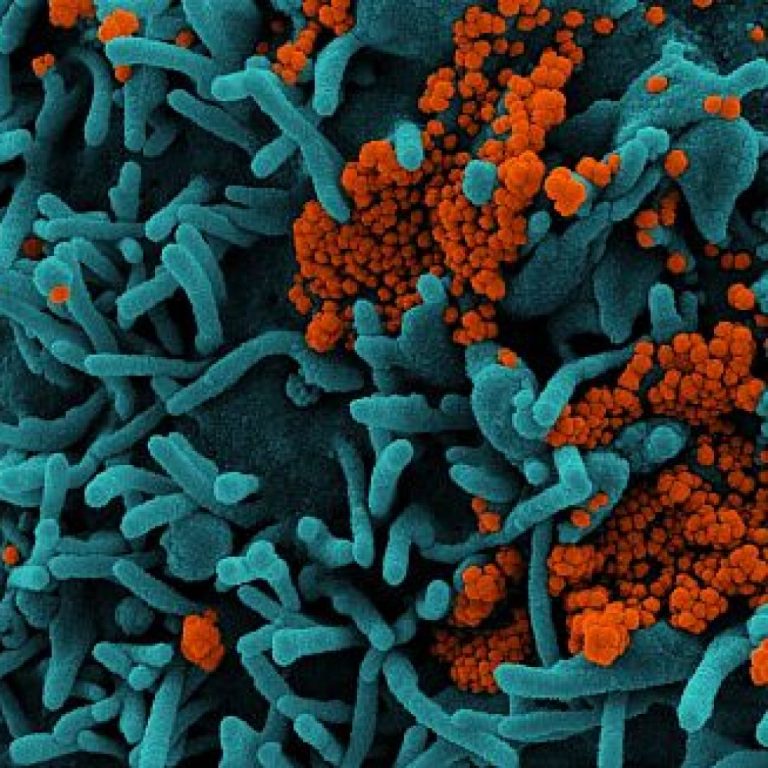
On Feb. 16, 2023, it was reported in the Lancet that protection from past infection against re-infection from…

On Feb. 16, 2023, National Institutes of Health (NIH) scientists announced they had developed and released an innovative…
On Feb. 15, 2023, the Cancer Prevention and Research Institute of Texas (CPRIT) announced a major step forward…
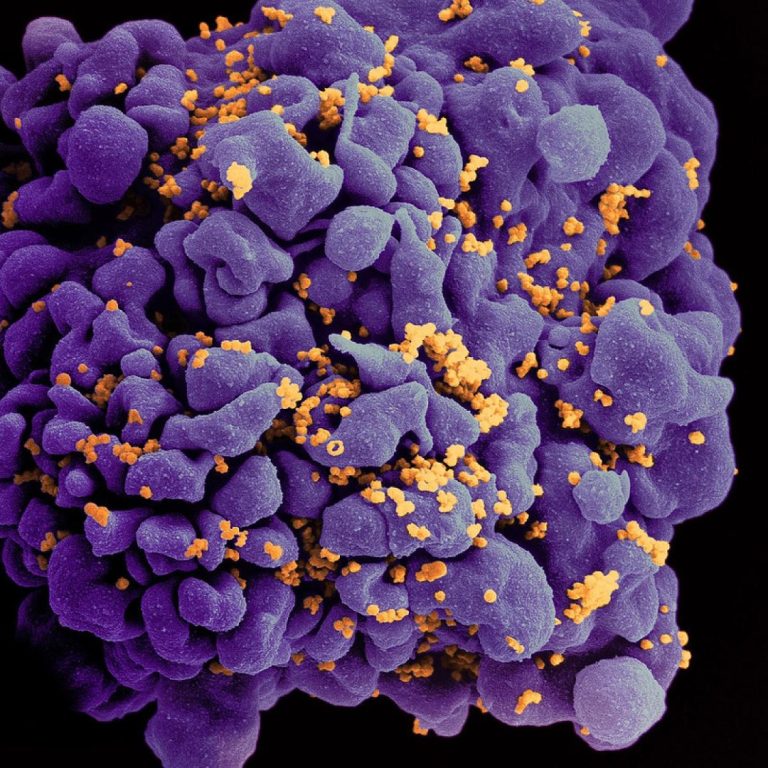
On Feb. 15, 2023, it was reported that a 53-year-old man in Germany had become at least the…

On Feb. 15, 2023, Moderna announced it will continue to offer its COVID-19 vaccines for free, even after…

On Feb. 14, 2023, the Pacific Economic Development Agency of Canada (PacifiCan), announced $14.5 million in funding for…

On Feb. 13, 2023, Novavax announced a modification to its existing agreement with the U.S. Department of Health…

On Feb. 10, 2023, the U.S. Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for…

On Feb. 9, 2023, the National Institutes of Health (NIH) announced that a new large-scale genetic analysis found…

On Feb. 8, 2023, as bird flu continues to circle the globe, a new report suggested that the…
On Feb. 8, 2023, BD announced that it had received Emergency Use Authorization from the U.S. Food and…