Emergent BioSolutions awarded 10-Year BARDA contract valued at $704 million for Ebanga treatment for Ebola
On Jul. 31, 2023, Emergent BioSolutions announced that it was awarded a 10-year contract by the Biomedical Advanced…
On Jul. 31, 2023, Emergent BioSolutions announced that it was awarded a 10-year contract by the Biomedical Advanced…
On Jul. 31, 2023, scientists at Washington University in St. Louis have developed a breath test that quickly…
On Jul. 28, 2023, the World Health Organization (WHO) reported circulating vaccine-derived poliovirus type 2 (cVDPV2) in the…

On Jul. 28, 2023, in a first-of-its-kind clinical trial, bioelectronic medicine researchers, engineers and surgeons at Northwell Healthメs…
On Jul. 28, 2023, the U.S. Food and Drug Administration (FDA) approved Merck’s Ervebo, a vaccine for the…
On Jul. 28, 2023, researchers from the Institute of Zoology at the University of Cologne, the Max Planck…

On Jul. 27, 2023, the California Institute for Regenerative Medicine (CIRM) awarded $50.1 million to fund clinical-stage research…

On Jul. 27, 2023, the IHR National Focal Point of Poland notified WHO of unusual deaths in cats…

On Jul. 27, 2023, SIGA Technologies announced that the U.S. Department of Health and Human Services exercised procurement…
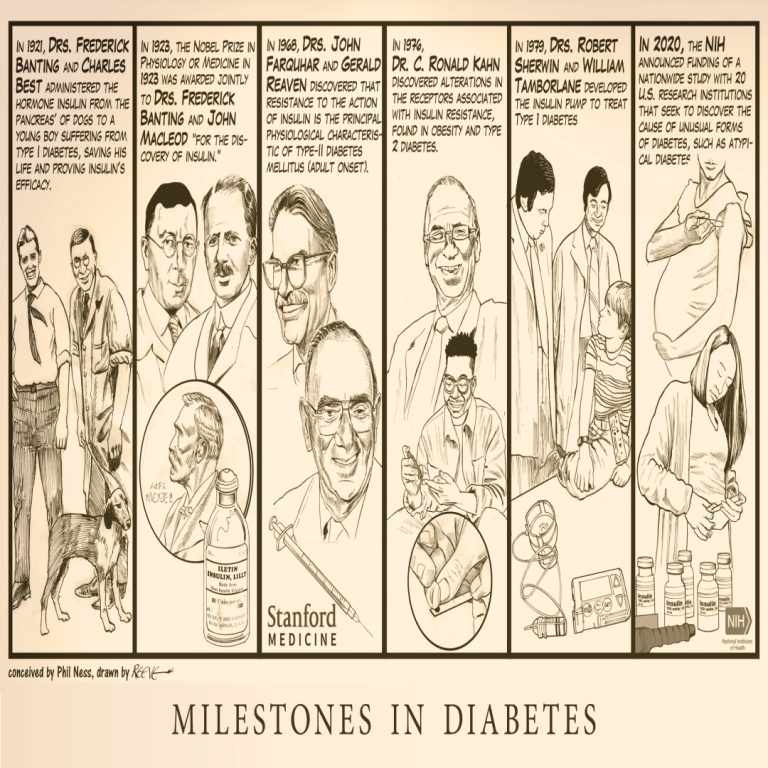
Milestones in Diabetes illustrates major milestones from the first administration of the hormone insulin in 1921 to a…

On Jul. 24, 2023, the Journal JAMA reported there is evidence that Republican-leaning counties had higher COVID-19 death…
On Jul. 24, 2023, the United Arab Emirates (UAE), notified WHO of a case of Middle East Respiratory…
On Jul. 19, 2023, the King County Department of Health issued a Health Advisory that Candida auris (C….

On Jul. 19, 2023, the National Institute of Health announced that a nationwide research team had created the…

On Jul. 14, 2023, Gilead Sciences announced that the U.S. Food and Drug Administration (FDA) had approved a…

On Jul. 14, 2023, the UK Animal and Plant Health Agency reported that additional asymptomatic human detections of…

On Jul. 13, 2023, Perrigo announced that the U.S. Food and Drug Administration had approved Opill, a progestin-only…

On Jul. 6, 2023, Eisai and Biogen announced that the U.S. Food and Drug Administration (FDA) had approved the…

On Jul. 6, 2023, University of Illinois Urbana-Champaign researchers reported they can detect exposure to the SARS-CoV-2 virus…

On Jul. 5, 2023, the IHR National Focal Point of Poland notified WHO of unusual deaths in cats…

On Jun. 29, 2023, the U.S. Food and Drug Administration approved Roctavian, an adeno-associated virus vector-based gene therapy…
On Jun. 27, 2023, researchers from Tel Aviv University announced they have developed an innovative gene therapy that…

On Jun. 27, 2023, the U.S. Centers for Disease Control and Prevention (CDC) adopted the 2023-2024 Advisory Committee…

On Jun. 26, 2023, Jaguar Health announced that the Take C.H.A.R.G.E. (Canine Health And ReGistry Exchange) had joined…

On Jun. 22, 2023 AquaBounty Technologies announced that the Company will pause the construction of its farm in…

On Jun. 22, 2023, the Institute for Health Metrics and Evaluation (IHME) reported that more than half a…

On Jun. 22, 2023, the U.S. Food and Drug Administration (FDA) approved Sarepta Therapeutics’s Elevidys, the first gene…

On Jun. 17, 2023, the Mississippi man known as “Case 1,” the first person to be diagnosed with…

On Jun. 16, 2023, the U.S. Food and Drug Administration’s (FDA) Vaccines and Related Biological Products Advisory Committee…
On Jun. 6, 2023, the U.S. Food and Drug Administration (FDA) granted granted marketing authorization for the Cue COVID-19…