U.S. Centers for Disease Control and Prevention warned of E. Coli outbreak linked to raw milk cheese
On Feb. 16, 2024, the U.S. Centers for Disease Control and Prevention announced that ten people infected with…
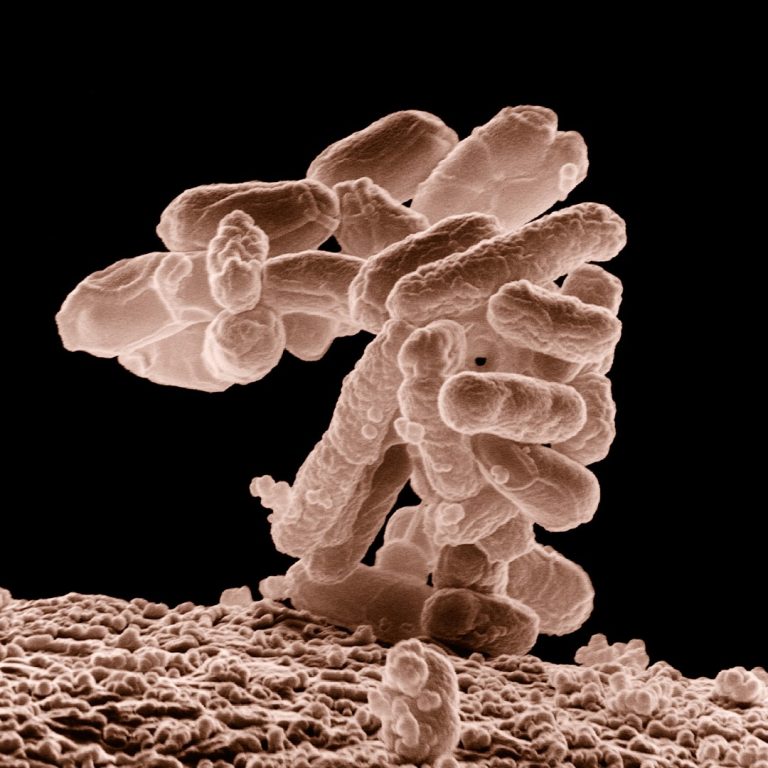
On Feb. 16, 2024, the U.S. Centers for Disease Control and Prevention announced that ten people infected with…

On Feb. 13, 2024, CRISPR Therapeutics announced that the European Commission had granted conditional marketing authorization to CASGEVY…

On Feb. 12, 2024, the U.S. Centers for Disease Control and Prevention (CDC) announced that Cambodia had reported…

On Feb. 9, 2024, the state of Alaska Department of Health Alaska Department of Health confirmed the first…

On Feb. 8, 2024, Deschutes County Health Services in Bend, Oregon confirmed a case of human plague in…
On Feb. 5, 2024, CSL and and Arcturus Therapeutics announced the results of a follow-up analysis of a…

On Feb. 2, 2024, Chinese scientists announced they have assembled the world’s most detailed human genome yet, a…

On Jan. 31, 2024, a U.S. Centers for Disease Control and Prevention (CDC) co-authored study published in Influenza…

On Jan. 25, 2024, a study led by the University of Edinburgh, is the largest genetic study of…

On Jan. 25, 2024, dozens of noted scientists, philanthropists, and Washington University, state and local leaders celebrated the…
On Jan. 24, 2024, Oregon Health & Science University announced that Pamela Runner, 77, of Corvallis, became the…

On Jan. 22, 2024, the University of California, Davis announced the first reported outbreak of high pathogenicity H5N1…

On Jan. 16, 2024, the U.S. Food and Drug Administration (FDA) announced it had approved CRISPR Therapeutics’ CASGEVY…

On Jan. 15, 2024, CSL announced that Swissmedic had authorised HEMGENIX® (etranacogene dezaparvovec), the first and currently only gene…

On Jan. 10, 2024, an National Institutes of Health (NIH) supported study announced an n atlas revealing the…

On Jan. 9, 2024, biologists in Alaska have confirmed the first case of a polar bear dying after…

On Jan. 9, 2024, Biogen and Eisai announced that humanized anti- soluble aggregated amyloid-beta (Aβ) monoclonal antibody ‘LEQEMBI’…

On Jan. 5, 2024, the U.S. Food and Drug Administration (FDA) authorized Florida’s Agency for Health Care Administration’s…

On Jan. 4, 2024, France announced it had detected bird flu on a duck farm in the Vendee…

On Jan. 4, 2024, AstraZeneca Sanofi’s Beyfortus (nirsevimab), a long-acting monoclonal antibody, was approved in China for the…

On Dec. 21, 2023, CSL and and Arcturus Therapeutics announced the results of a Phase 3 study showing…

On Dec. 21, 2023, the World Health Organization (WHO) announced that it had prequalified the R21/Matrix-M malaria vaccine…
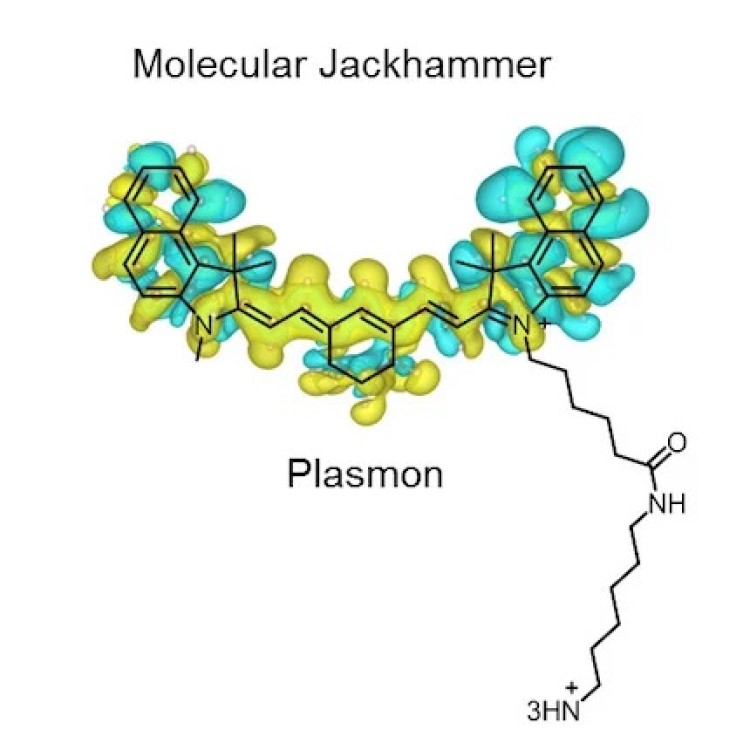
On Dec. 19, 2023, a research team led by Rice University reported they found that the atoms of…

On Dec. 19, 2023, the U.S. Food and Drug Administration approved the first test that uses DNA in…

On Dec. 18, 2023, the Novo Nordisk Foundation announced it was committing up to DKK 1.8 billion (USD…

On Dec. 18, 2023, Novavax announced that the Taiwan Food and Drug Administration had granted emergency use authorization…

On Dec. 18, 2023, the U.S. Centers for Disease Control and Prevention (CDC) announced a study that showed…

On Dec. 14, 2023, the Department of Energy’s (DOE) Pacific Northwest National Laboratory (PNNL) announced that it had…

On Dec. 14, 2023, the University of Alberta announced that a study published in Nature sheds light on…

On Dec. 14, 2023, Pfizer announced it had completed the acquisition of Seagen, a global biotechnology company based…