BioNTech sued by UPenn over COVID-19 vaccine patent royalties
On Aug. 6, 2024, the University of Pennsylvania announced it had sued German biotechnology firm BioNTech n Pennsylvania…

On Aug. 6, 2024, the University of Pennsylvania announced it had sued German biotechnology firm BioNTech n Pennsylvania…

On Aug. 5, 2024, North Carolina State University researchers revealed that an examination of the genetic material found…
On Aug. 5, 2024, Novartis and Viatris were named a federal lawsuit in Maryland by the family of…
On Aug. 2, 2024, the U.S. Food and Drug Administration approved Adaptimmune’s Tecelra (afamitresgene autoleucel), a gene therapy…
On Aug. 1, 2024, the Van Andel Institute reported that humans and baker’s yeast have more in common…

On Aug. 1, 2024, the Coalition for Epidemic Preparedness Innovations (CEPI) and the World Health Organization (WHO) called…

On Jul. 31, 2024, researchers at Washington State University (WSU) announced that the cannabigerol (CBG) effectively reduced anxiety…

On Jul. 31, 2024, a large study led by researchers at the American Cancer Society (ACS) suggested incidence…
On Jul. 30, 2024, a head-to-head comparison of six commercially available blood tests for Alzheimer’s disease led by…

On Jul. 29, 2024, a team led by the Barbara Ann Karmanos Cancer Institute and the Wayne State…

On Jul. 29, 2024, a new project that aims to accelerate the development and accessibility of human avian…

On Jul. 26, 2024, the U.S. Food and Drug Administration (FDA) approved the Guardant Health’s Shield, a test…

On Jul. 26, 2024, the Maryland Department of Health advised consumers not to eat certain deli meats produced…

On Jul. 26. 2024, Boar’s Head, a Jarratt, Va., establishment, announced it was recalling all liverwurst product produced…

On Jul. 25, 2024, Pfizer announced that the European Commission (EC) has granted conditional marketing authorization for DURVEQTIX®…

On Jul. 25, 2024, doctors from the Texas Heart Institute (THI), BiVACOR®, Baylor St. Luke’s Medical Center and…
On Jul. 25, 2024, the World Health Organization (WHO) announced during the 25th International AIDS Conference, being held…

On Jul. 25, 2024, the California Institute for Regenerative Medicine (CIRM), one of the world’s largest institutions dedicated…

On Jul. 25, 2024, the Government of Hong Kong reported that H5N6 avian flu had infected a 70-year-old…

On Jul. 24, 2024, the University of Hong Kong released a study that showed hospitalized COVID-19 patients treated…
On Jul. 22, 2024, scientists at ADA Forsyth Institute (AFI) announced they had identified a critical factor that…

On Jul. 24, 2024, a team led by researchers at Cornell University announced a study that provides evidence…

On Jul. 23, 2024, a study published in Cell Reports revealed that seabirds likely facilitated the spread of…
On Jul. 19, 2024, the Centers for Disease Control and Prevention (CDC) announced that it had begun an…
On Jul. 19, 2024, a study led by the University of Oxford reported that a group of small…
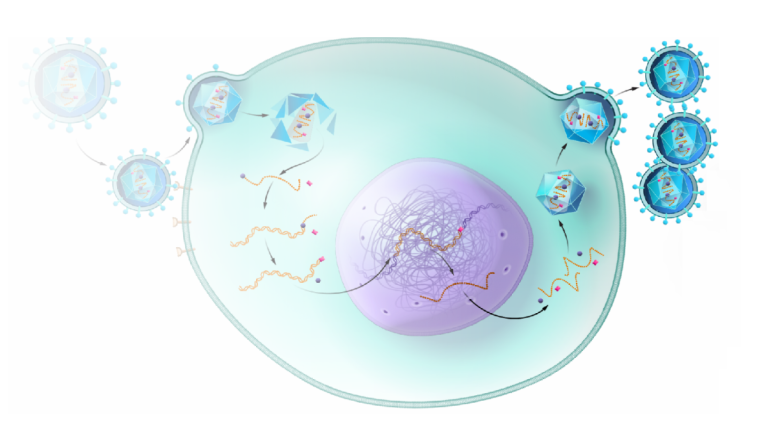
On Jul. 17, 2024, the National Cancer Institute (NCI) announced that a study, funded in part by the…

On Jul. 16, 2024, researchers at the National Institutes of Health (NIH) reported they have found that taking…
On Jul. 15, 2024, the Sabin Vaccine Institute launched a Phase 2 clinical trial for its vaccine against…

On Jul. 11, 2024, a team led by scientists from Baylor College of Medicine, University of Copenhagen, and…

On Jul. 11, 2024, Stéphane Peyrégne from the Max Planck Institute for Evolutionary Anthropology unveiled a genetic sequence…