Human skin map gives ‘recipe’ to build skin and could help prevent scarring
On Oct. 16, 2024, researchers from the Wellcome Sanger Institute, Newcastle University and their collaborators announced they had…
On Oct. 16, 2024, researchers from the Wellcome Sanger Institute, Newcastle University and their collaborators announced they had…

On Oct. 16, 2024, Know Labs in Seattle announced that it had retained The Stanbridge Group to secure…

On Oct. 16, 2024, Sanofi announced it will contribute $18 million to three Historically Black Medical Schools to…
On Oct. 16, 2024, a National Institutes of Health (NIH)-funded clinical trial of an mpox vaccine in adolescents…

On Oct. 15, 2024, Massachusetts Governor Maura Healey and the Massachusetts Life Sciences Center (MLSC) announced $21.4 million…

On Oct. 14, 2024, researchers have found a link between two human-specific genes and the gene SYNGAP1, a…

On Oct. 14, 2024, Lundbeck and Longboard Pharmaceuticals announced an agreement for Lundbeck to acquire Longboard for payment…

On Oct. 14, 2024, the Global Preparedness Monitoring Board (GPMB) released a report that outlined 15 key drivers…
On Oct, 14, 2024, a team of researchers from the Allen Institute of Brain Science and the University…

On Oct. 14, 2024, RedHill Biopharma announced that the U.S. government’s Biomedical Advanced Research and Development Authority (BARDA)…
On Oct. 12, 2024, the Sabin Vaccine Institute announced it had delivered approximately 1,000 more investigational vaccine doses…
On Oct. 11, 2024, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved HYMPAVZI™ (marstacimab-hncq)…

On Oct. 11, 2024, a team of researchers from University of Illinois Urbana-Champaign announced DNA analysis of the…
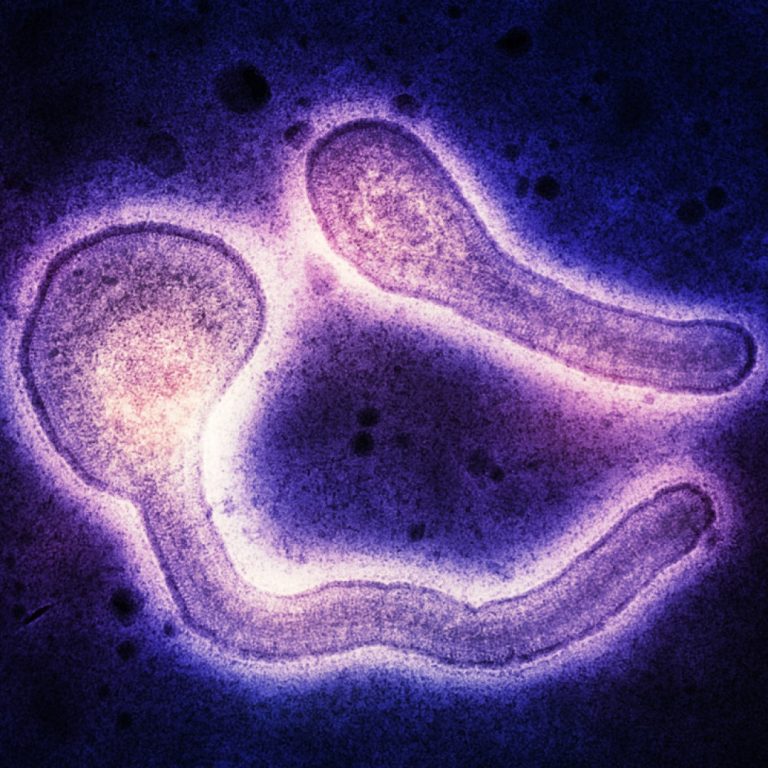
On Oct. 11, 2024, the World Health Organization (WHO) reported that a total of 58 cases of Marburg…
On Oct. 11, 2024, scientists at the Korea Advanced Institute of Science and Technology (KAIST) announced they had…

On Oct. 11, 2024, researchers in China published a study that provided details about a new wetland virus….

On Oct. 10, 2024, health officials from the U.S. Centers for Disease Control and Prevention announced they had…
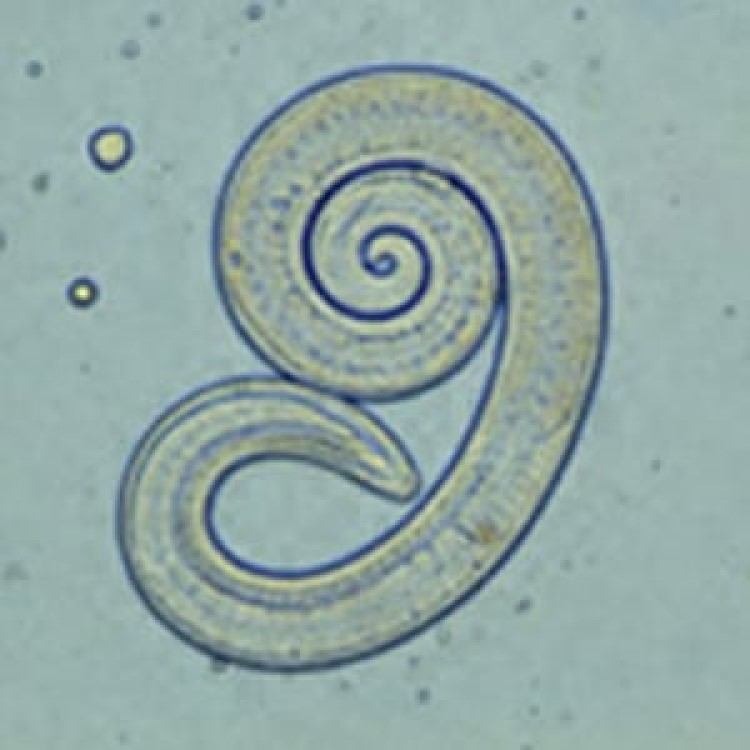
On Oct. 10, 2024, the U.S. Centers for Disease Control and Prevention announced that a 2023 presumed outbreak…

On Oct. 10, 2024, a report by the World Health Organization (WHO) found that vaccines against 24 pathogens…
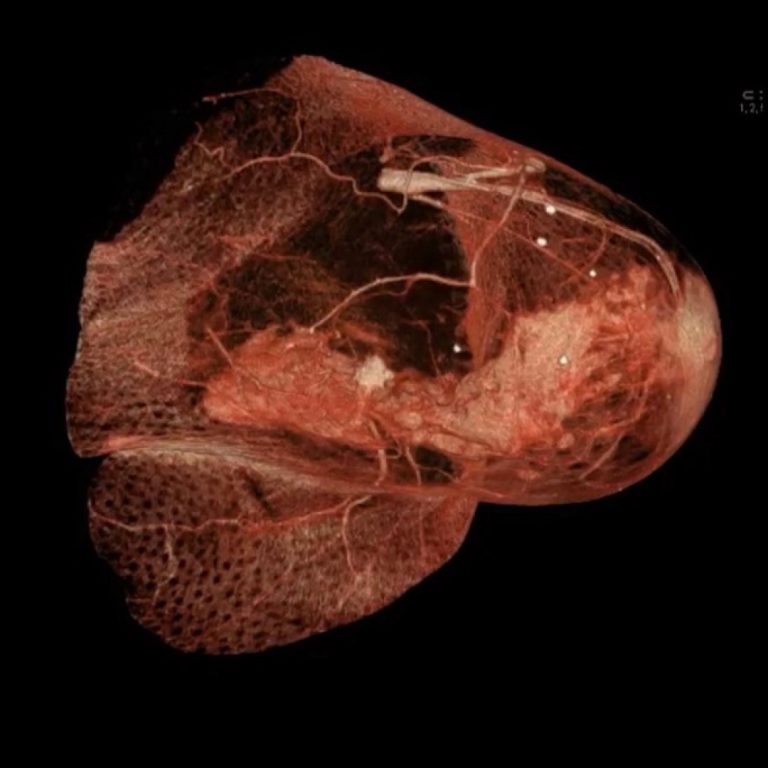
On Oct. 10, 2024, Genentech announced today that the U.S. Food and Drug Administration (FDA) approved ItovebiTM (inavolisib),…
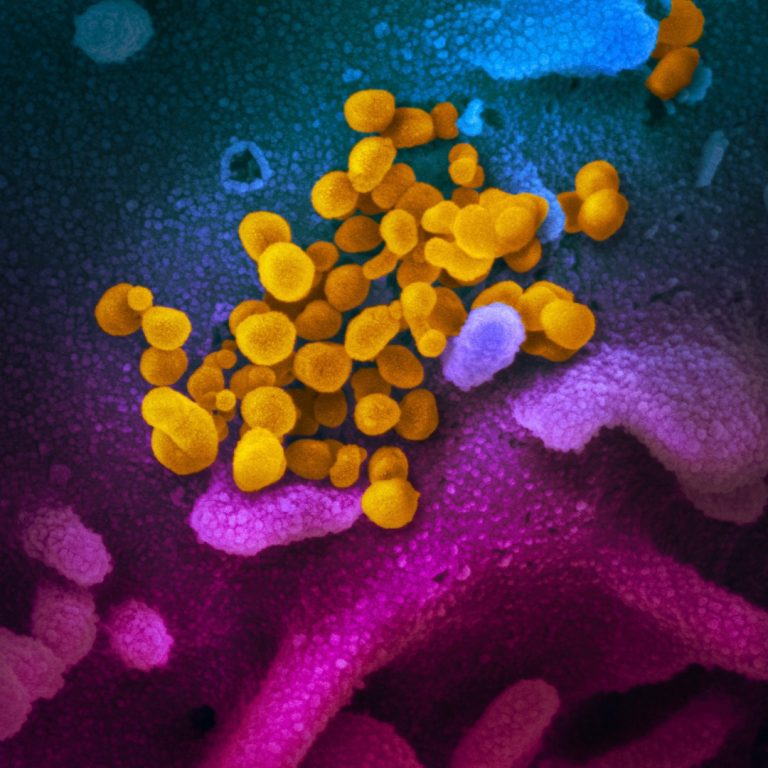
On Oct. 9, 2024, researchers from Brigham and Women’s Hospital found that people with wide-ranging long COVID symptoms…
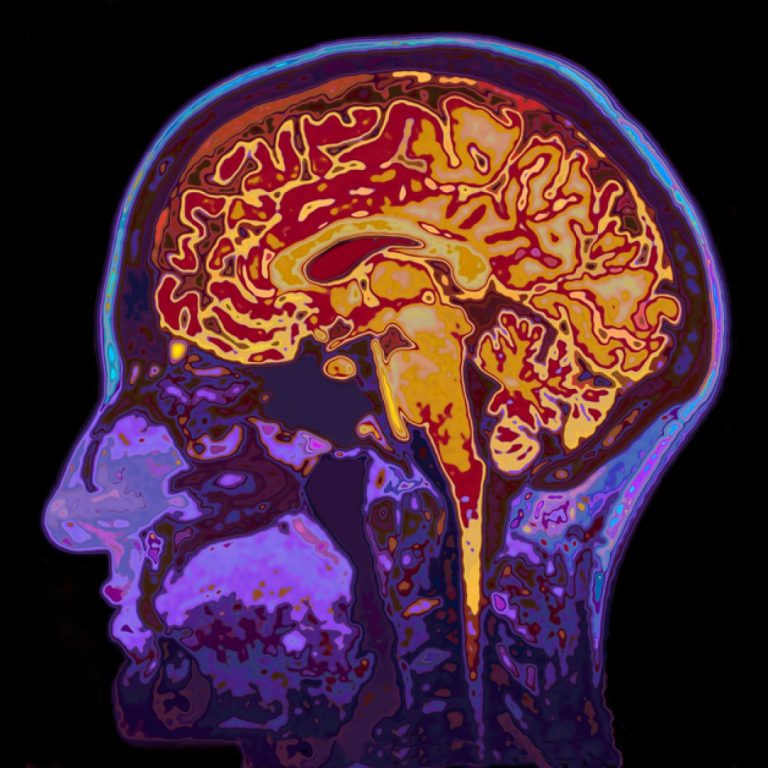
On Oct. 9, 2024, scientists from Oregon Health & Science University (OHSU) released a study that used imaging…

On Oct. 9, 2024, in a Northwestern University-led study, microbiologists found that showerheads and toothbrushes were teeming with…

On Oct. 9, 2024, the Nobel Foundation awarded the Nobel Prize in Chemistry: one half to David Baker,…

On Oct. 9, 2024, Novavax announced that the European Commission had granted Marketing Authorization for Novavax’s updated 2024-2025…

Oct. 9, 2024, the Seattle Fire Department (SFD) expanded its Buprenorphine Pilot Program and Seattle became the first…

On Oct. 8, 2024, the Pan American Health Organization (PAHO) announced an epidemiological alert and urged countries in…

On Oct. 8, 2024, a team of University of Essex researchers found that the secret to losing weight…

On Oct. 8, 2024, researchers from the Max Planck Institute for Plant Breeding announced they have developed a…

On Oct. 8, 2024, scientists from the Commonwealth Scientific and Industrial Research Organisation (CSIRO), Australia’s national science agency,…