Human Cell Atlas achieved leap in understanding of the human body
On Nov. 20, 2024, researchers with the global Human Cell Atlas (HCA) consortium reported significant progress in their…

On Nov. 20, 2024, researchers with the global Human Cell Atlas (HCA) consortium reported significant progress in their…

On Nov. 20, 2024, a research collaboration between the Texas A&M College of Veterinary Medicine and Biomedical Sciences…

On Nov. 19, 2024, the California Department of Public Health (CDPH) reported it has identified a possible bird…
On Nov. 19, 2024, the World Health Organization (WHO) announced it had granted Emergency Use Listing (EUL) to Japan-based…
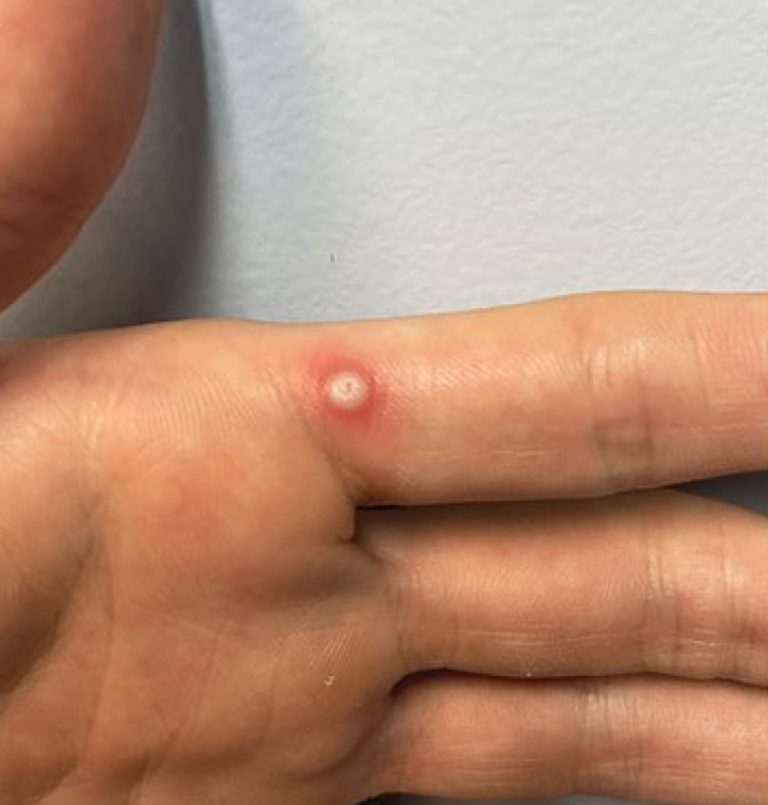
On Nov. 16, 2024, the California Department of Public Health (CDPH) announced it had identified through laboratory testing…
On Nov. 16, 2024, the U.S. Centers for Disease Control and Prevention (CDC) and public health officials in…
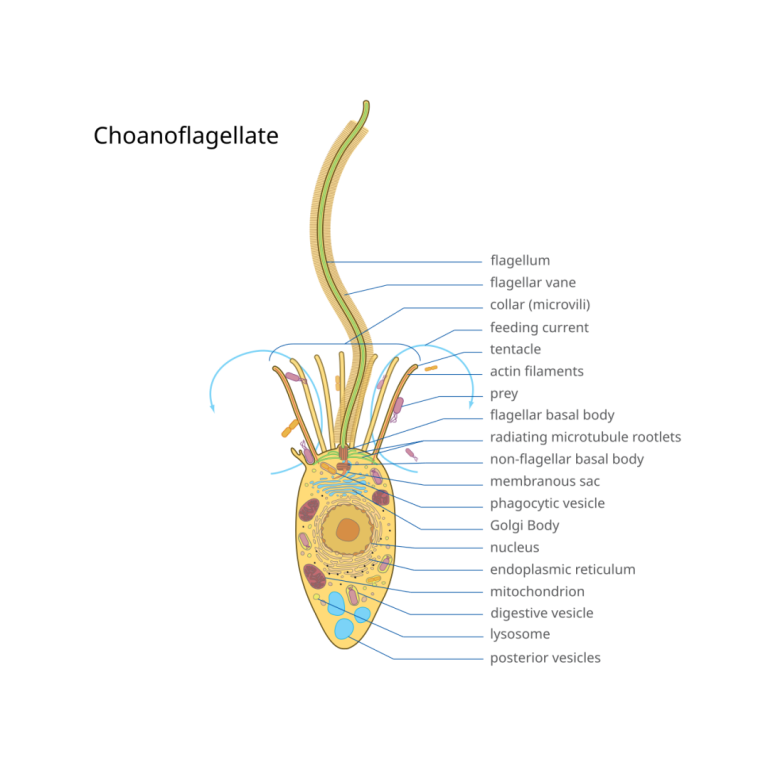
On Nov. 15, 2024, researchers from Queen Mary University of London and the the University of Hong Kong…

On Nov. 15, 2024, the White House Office of Science and Technology Policy released a report on Building…
On Nov. 15, 2024, a University of California Davis Health (UC Davis Health) research team announced they had…

On Nov. 15, 2024, a team led by researchers from Sweden’s Karolinska Institutet reported study results that showed…

On Nov. 15, 2024, the AARP, the Alzheimer’s Disease Data Initiative (AD Data Initiative), and the Institute for…

On Nov. 15, 2024, the U.S. Centers for Disease Control and Prevention (CDC) confirmed a highly pathogenic avian…
On Nov. 15, 2024, an international research team including scientists from the Max Planck Institute reported that critical…

On Nov. 15, 2024, The Hawai‘i Department of Agriculture (HDOA) received confirmation from the U.S. Department of Agriculture…

On Nov. 14, 2024, officials at the Woodland Park Zoo in Seattle announced that a red-breasted goose had…

On Nov. 14, 2024, the March of Dimes released its 2024 Report Card, revealing the U.S. preterm birth…

On Nov. 14, 2024, the World Health Organization (WHO) and the U.S. Centers for Disease Control and Prevention…

On Nov. 14, 2024, the U.S. Food and Drug Administration approved PTC Therapeutics’ Kebilidi (eladocagene exuparvovec-tneq), an adeno-associated…
On Nov. 14, 2024, an analysis from the Global Burden of Disease Study Collaborator Network warned of an…

On Nov. 13, 2024, the U.S. Food and Drug Administration (FDA) and the U.S. Centers for Disease Control…

On Nov. 13, 2024, the NCD Risk Factor Collaboration (NCD-RisC) with support from the World Health Organization (WHO)…

On Nov. 13, 2024, a study published in JAMA Network Open showed that respiratory syncytial virus (RSV) was…

On Nov. 12, 2024, researchers from the University of Basel and University Hospital Basel announced they had developed…

On Nov. 12, 2024, researchers from the University of California, San Francisco (UCSF) reported that their genomic test…
On Nov. 12, 2024, researchers led by Nico Sommerdijk from Radboud university medical center in Nijmegen (Netherlands) announced…
On Nov. 12, 2024, a research team led by UCLA Health Jonsson Comprehensive Cancer Center investigators has shown that…
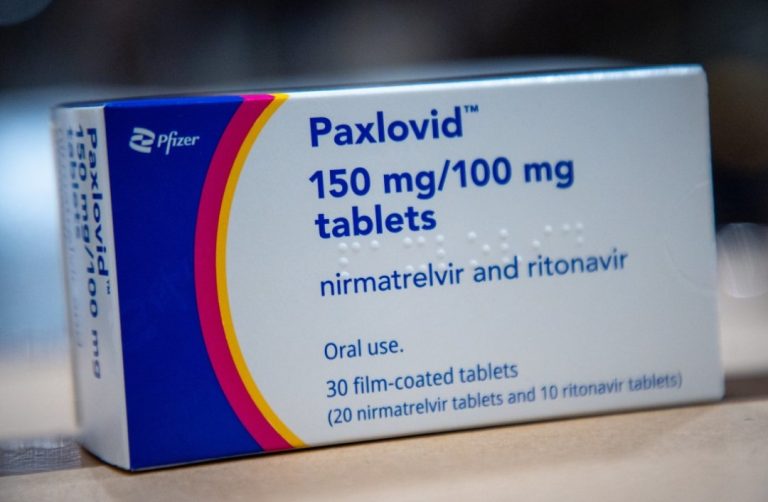
On Nov. 11, 2024, findings from two studies have tied use of the antiviral drug nirmatrelvir-ritonavir (Paxlovid) to…

On Nov. 11, 2024, a team of researchers led by Iowa State University (ISU) have shown that as…
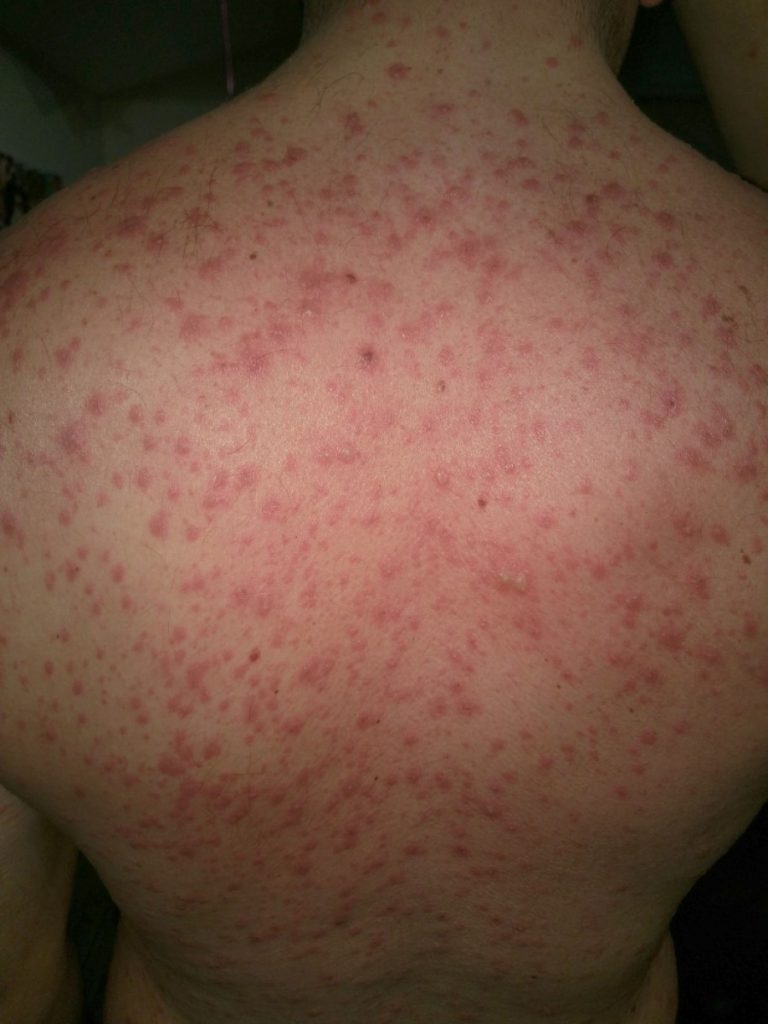
On Nov. 11, 2024, an international team of researchers has for the first time cured patients with Toxic…

On Nov. 11, 2024, a study published in the journal Nature Communications and co-led by UC Davis and…