Scientists Identify Alzheimer’s Disease-Protective Genetic Factors and Unravel Disease Mechanisms
On Feb. 4, 2025, an international research teams led by Hong Kong University of Science and Technology (HKUST)…

On Feb. 4, 2025, an international research teams led by Hong Kong University of Science and Technology (HKUST)…
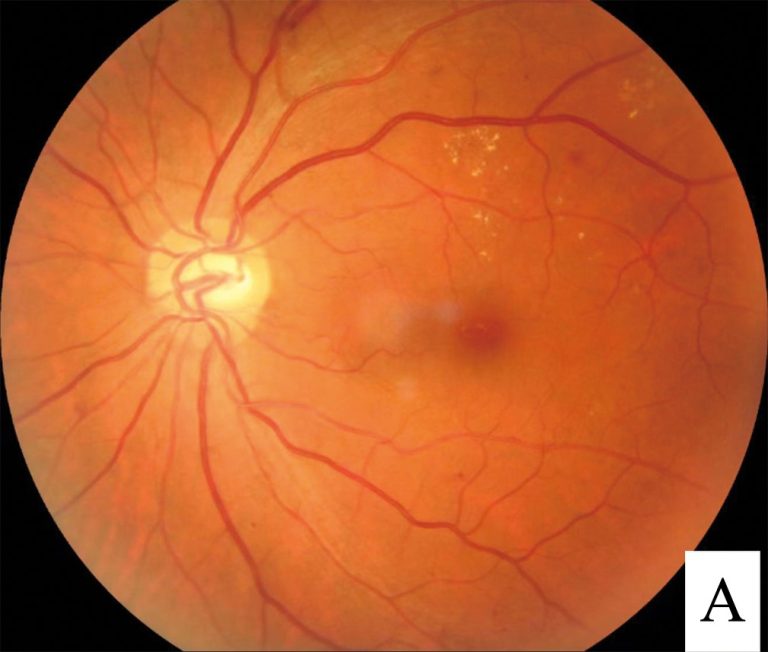
On Feb. 4, 2025, Genentech announced that the U.S. Food and Drug Administration (FDA) had approved Susvimo® (ranibizumab…
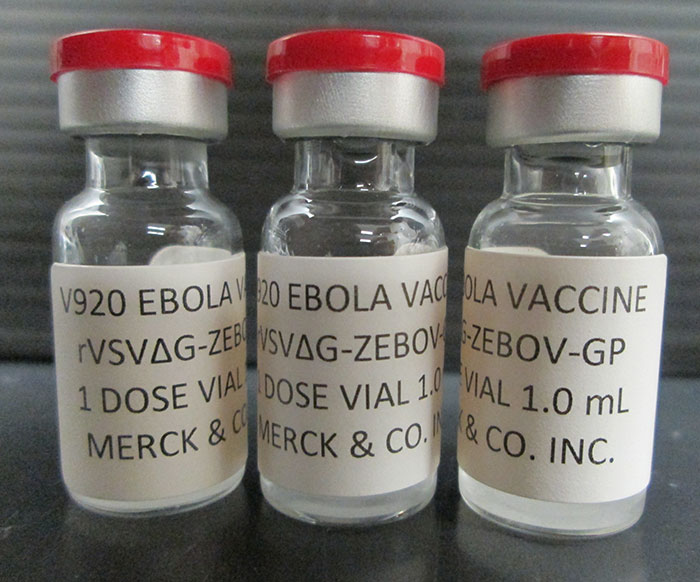
On Feb. 3, 2025, in a global first, Uganda’s Ministry of Health, the World Health Organization (WHO) and…
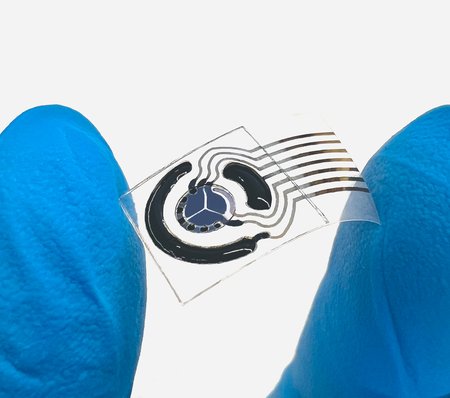
On Feb. 3, 2025, a team of Caltech engineers announced they have developed a technique for inkjet printing…
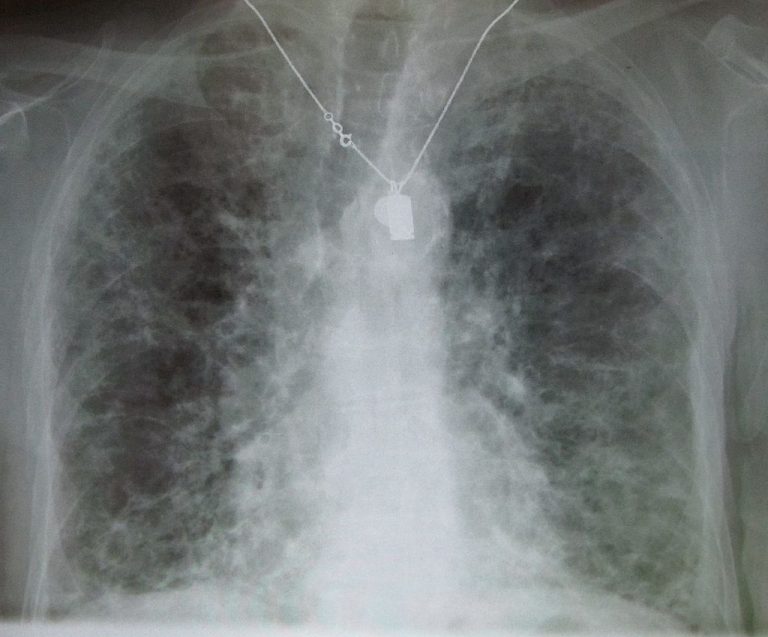
On Feb. 3, 2025, a team of researchers co-led by TGen, part of City of Hope announced creating…

On Feb. 3, 2025, United Therapeutics, a public benefit corporation, announced that the U.S.Food and Drug Administration (FDA)…

On Feb. 3, 2025, scientists from Montana State University reported new knowledge of how ancient microorganisms adapted from…
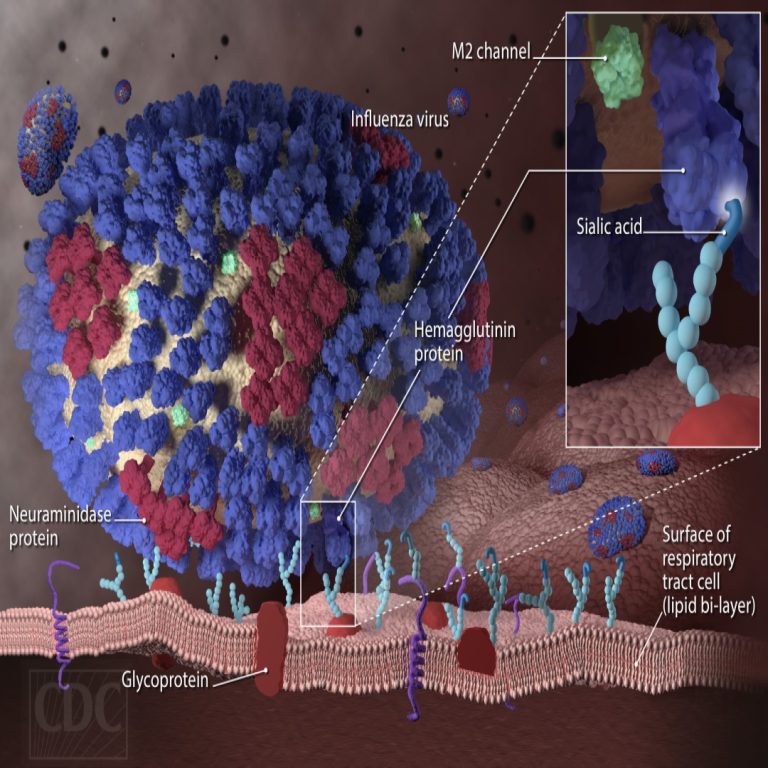
On Jan. 31, 2025, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) confirmed…
On Jan. 31, 2025, the Kansas Department of Health and Environment (KDHE) reported that there were 67 confirmed…

On Jan. 30, 2025, the California Institute for Regenerative Medicine (CIRM) announced it has awarded nearly $100 million…

On Jan. 30, 2025, the World Health Organization (WHO) announced it was mobilizing efforts to support national health…

On Jan. 30, 2025, the World Health Organization (WHO) congratulated Niger for having met the criteria for onchocerciasis…

On Jan. 30, 2025, the U.S. Food and Drug Administration (FDA) approved Vertex Pharmaceuticals’s Journavx (suzetrigine) 50 milligram…
On Jan. 29, 2025, researchers from the University Medical Center Göttingen announced they have shown that patches of…
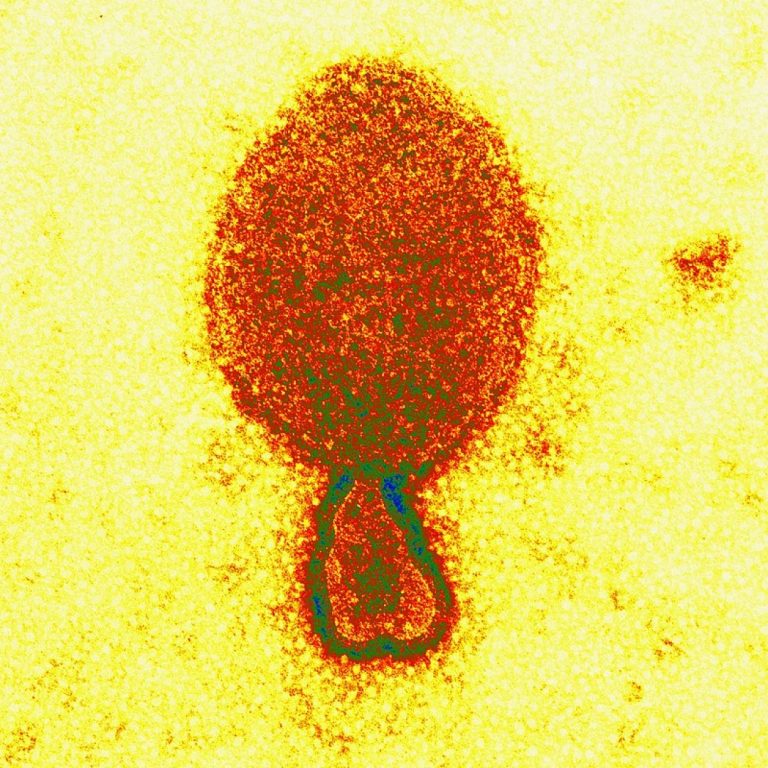
On Jan. 29, 2025, researchers at The University of Queensland announced they have identified the first henipavirus in…

Jan. 29, 2025, an international team of researchers led by the Karolinska Institutet announced they have mapped the…

On Jan. 28, 2025, Novo Nordisk announced that the U.S. Food and Drug Administration (FDA) has approved Ozempic® to…

On Jan. 28, 2025, Rose Acre Farms, the nation’s second largest egg producer, reported that tests have confirmed…

On Jan. 27, 2025, researchers at the Broad Institute of MIT and Harvard, along with collaborators at Calico…

On Jan. 27, 2024, the World Organisation for Animal Health (WOAH) reported that highly pathogenic avian influenza (HPAI)…

On Jan. 27, 2025, a team of researchers from the University of Buffalo found that In the quest…
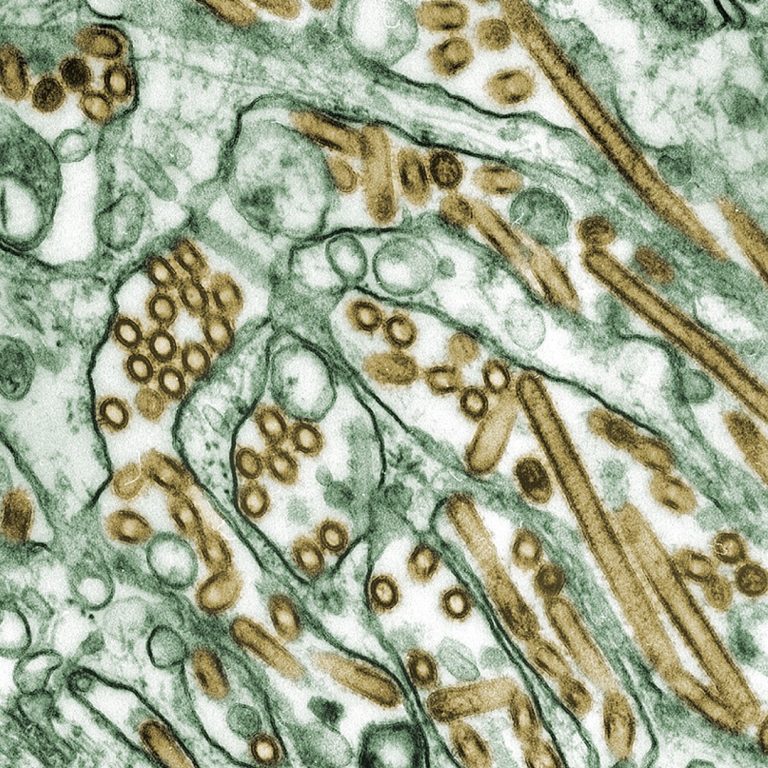
On Jan. 27, 2025, researchers reported that the H5N1 virus is adapting to new mammalian hosts, raising the…
Jan. 24, 2025, the U.S. Food and Drug Administration (FDA) pulled draft guidance from its website requiring companies…
On Jan. 24, 2025, the World Health Organization (WHO) reported that from January 1 to December, 29, 2024,…

On Jan. 24, 2025, researchers from the Veterans Affairs Portland Health Care System in Oregon reported results from…

On Jan. 24, 2025, The South Dakota Department of Health reported the death of a child due to…

On Jan. 23, 2025, the World Health Organization (WHO) announced that following a nearly century-long effort, Georgia has…

On Jan. 22, 2025, researchers from the University of Virginia School of Medicine reported revealing the fingerprints of…
On Jan. 22, 2025, researchers at Dana-Farber Cancer Institute announced they have developed a breakthrough method to detect…
On Jan. 22, 2025, scientists at deCODE genetics/Amgen announced they have constructed a complete map of how human…