CPRIT awards $68 million in research grants to Texas institutions
On Feb. 19, 2025, the Cancer Prevention and Research Institute of Texas (CPRIT) announced that it had approved…

On Feb. 19, 2025, the Cancer Prevention and Research Institute of Texas (CPRIT) announced that it had approved…

On Feb. 19, 2025, Alcon announced the full U.S. commercial availability of Voyager™ DSLT, the first and only…

On Feb. 19, 2025, research from the Texas A&M College of Veterinary Medicine and Biomedical Sciences has discovered…
On Feb. 19, 2025, scientists at St. Jude Children’s Research Hospital report results from a promising new approach…

On Feb. 18, 2025, a research expedition led by Antonio Alcamí at the Severo Ochoa Molecular Biology Center…

On Feb. 18, 2025, Hologic announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance…

On Feb. 18, 2025, scientists from the Institute for Systems Biology announced they have developed a breakthrough method…

On Feb. 17, 2025, researchers from Texas Children’s Hospital in Houston announced that the four year old girl…

On Feb. 17, 2025, Stanford researchers have conducted the first large-scale screen of these inherited changes, called single…

On Feb. 14, 2025, Zoetis announced that the U.S. Department of Agriculture (USDA), Center for Veterinary Biologics (CVB)…

On Feb. 14, 2025, the World Health Organization (WHO) announced that a cumulative of two confirmed and eight…
On Feb. 14, 2025, the Texas Department of State Health Services reported an outbreak of measles in the…

Feb. 14, 2025, the Wyoming Department of Health (WDH) reported that the state’s first case of H5N1 avian…

On Feb. 13, 2025, public health officials from the U.S. Centers for Disease Control and Prevention (CDC) reported…

On Feb. 13, 2025, Ralph L. Abraham, MD, the Louisiana Surgeon General announced that the state will no…
On Feb. 13, 2025, researchers at Washington University School of Medicine in St. Louis announced a new analysis…
On Feb. 12, 2025, the New York State Department of Health issued a Health Advisory to health care…
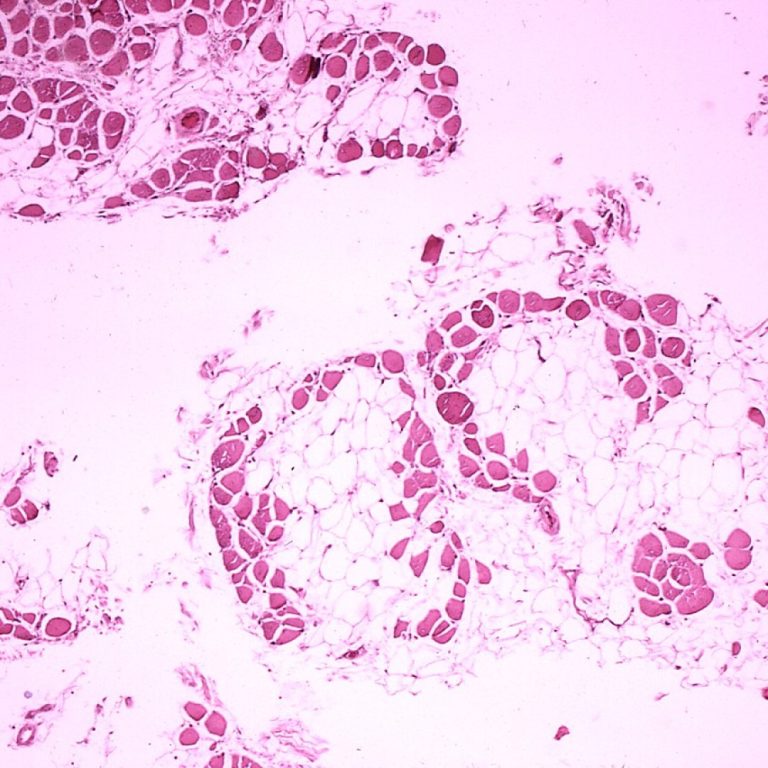
On Feb. 12, 2025, researchers at the Max Planck Institute released a study that could pave the way…

On Feb. 12, 2025, a study led by researchers from the University of Washington announced that a new…
On Feb. 12, 2025, Genentech announced that the U.S. Food and Drug Administration (FDA) has approved a New…
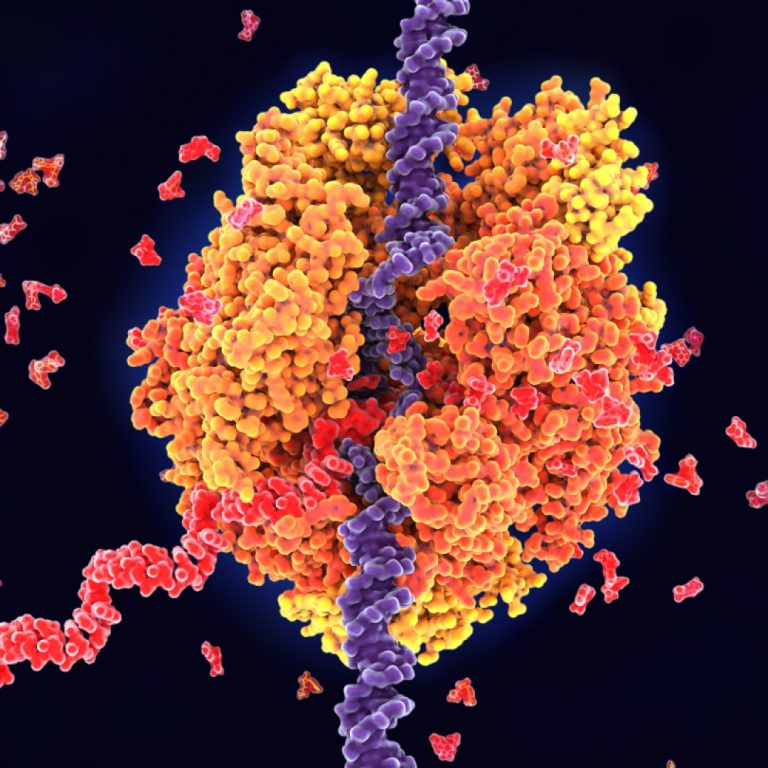
On Feb. 11, 2025, scientists from Ohio State University (OSU) have discovered that some tiny segments of RNA…

On Feb. 11, 2025, Novartis announced an agreement to acquire Anthos Therapeutics, a Boston-based, privately held, clinical-stage biopharmaceutical…
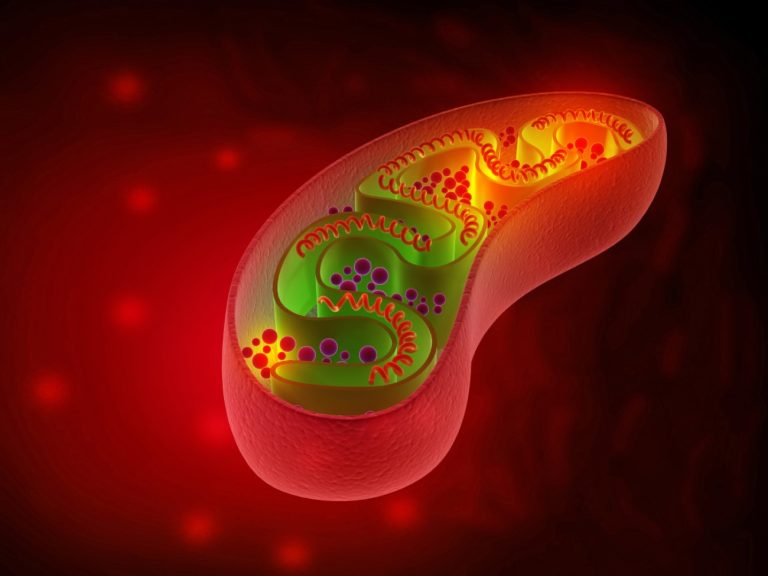
On Feb. 11, 2025, in study of mitochondrial disease at the University of Alberta researcher revealed an easier…
On Feb. 10, 2025, scientists announced identifying genetic variants that could have helped a man to avoid dementia…

On Feb. 8, 2025, the United States Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS)…

On Feb. 7, 2025, an international team of researchers reported Yersinia pestis genome had been recovered from a…

On Feb. 6, 2025, a team of synthetic biologists from Yale University announced they have re-written the genetic…

On Feb. 5, 2025, scientists from the Cancer Dependency Map (DepMap) at the Broad Institute of MIT and…

On Feb. 5, 2025, the proposed dismantling of the U.S. Agency for International Development (USAID) could disrupt clinical…
On Feb. 5, 2025, the Spokane Regional Health District announced the first confirmed death from pertussis (whooping cough)…