Two Deaths of rare brain disorder reported in Oregon
On Apr. 14, 2025, the Hood River County Health Department reported three cases of the rare brain disorder…
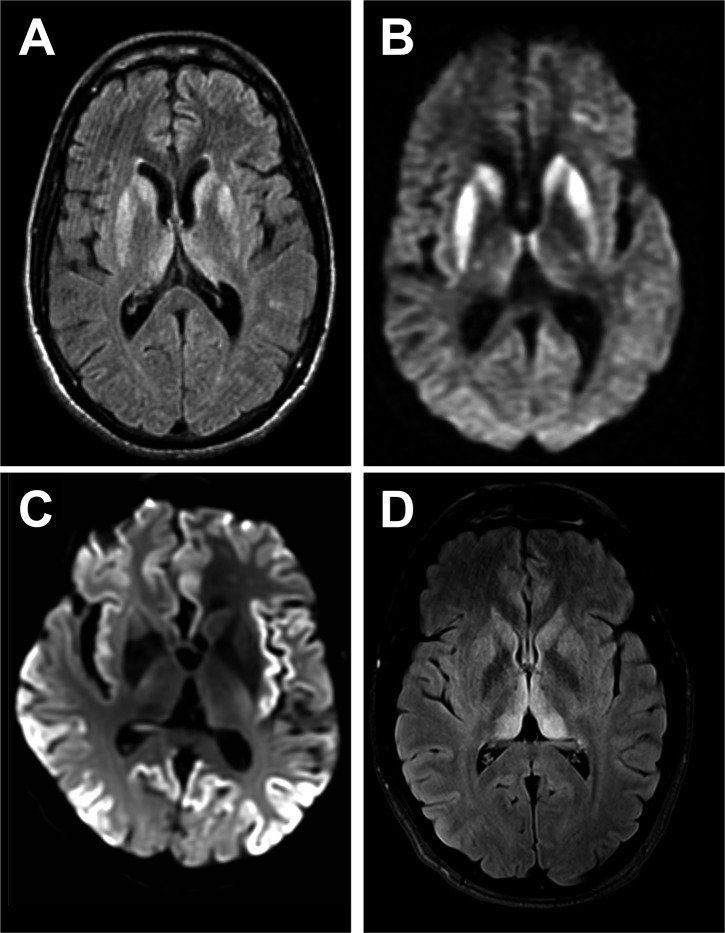
On Apr. 14, 2025, the Hood River County Health Department reported three cases of the rare brain disorder…
On Apr. 14, 2025, a new University of Southern California (USC) study suggests that gut imbalances in children…
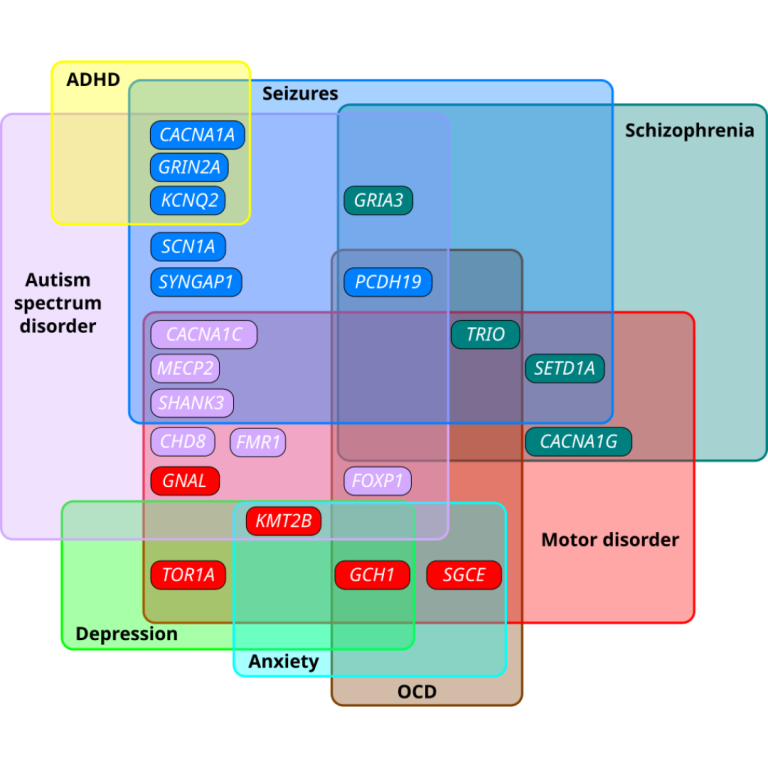
On Apr. 14, 2025, a research team at the Institute for Basic Science (IBS) in Korea announced they…

On Apr. 11, 2025, Africa’s top public health institution announced it is planning to tap more funds from…

On Apr. 11, 2025, the UK National Institute for Health and Care Excellence (NICE) recommended capivasertib (also called…
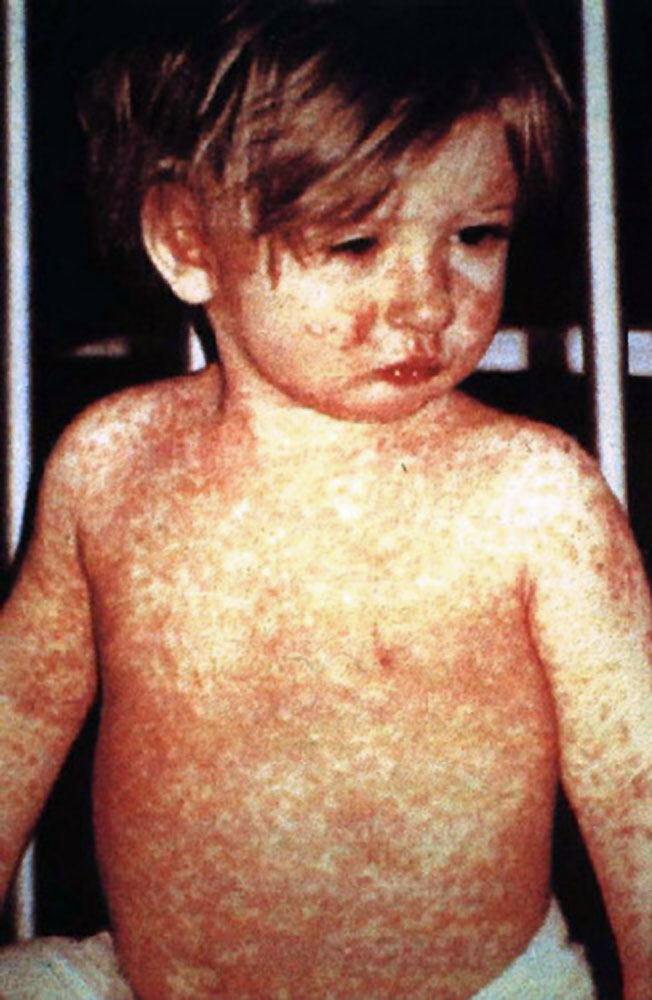
On Apr. 11, 2025, the Texas Department of State Health Services (Texas DSHS) reported that 541 cases of…
On Apr. 10, 2025, the World Health Organization (WHO) published its first-ever global guidelines for meningitis diagnosis, treatment…
On Apr. 10, 2025, the U.S. Food and Drug Administration announced it is taking a groundbreaking step to…

On Apr. 10, 2025, Novartis, a leading global innovative medicines company, announced a planned $23 billion investment over…

On Apr. 9, 2025, an international, multi-institutional team of researchers announced that comprehensive reference genomes have now been…
On Apr. 9, 2025, a team of neuro scientists scientists published findings from the Machine Intelligence from Cortical…

On Apr. 9, 2025, a research team from the University of Texas, San Antonio announced a study that…

On Apr. 9, 2025, Roswell Park Comprehensive Cancer Center achieved textbook outcomes for 90% of 150 consecutive robot-assisted,…

On Apr. 9, 2025, Tulane University researchers announced they have developed a first-of-its-kind handheld diagnostic device that can…

On Apr. 8, 2025, the National Security Commission on Emerging Biotechnology delivered its major report and action plan…

On Apr. 8, 2025, the Hawaiʻi Department of Health (DOH) State Laboratories Division confirmed a case of measles…

On Apr. 7, 2025, in a landmark study, researchers at University of Nebraska Medical Center (UNMC) announced they…
On Apr. 7, 2025, scientists at Colossal Biosciences announced the successful de-extinction of the dire wolf with the…

On Apr. 7, 2025, The Indiana Department of Health (IDOH) reported the first laboratory confirmed case of measles…

On Apr. 7, 2025, the Colorado Department of Public Health and Environment issued a Health Alert Network (HAN)…

On Apr. 6, 2025, the Texas Department of State Health Services has reported the second measles death of…

On Apr. 4, 2025, a 3-year-old girl from the western state of Durango is Mexico’s first confirmed human…

On Apr. 4, 2025, the Texas Department of State Health Services (DSHS) reported updated information about an outbreak…
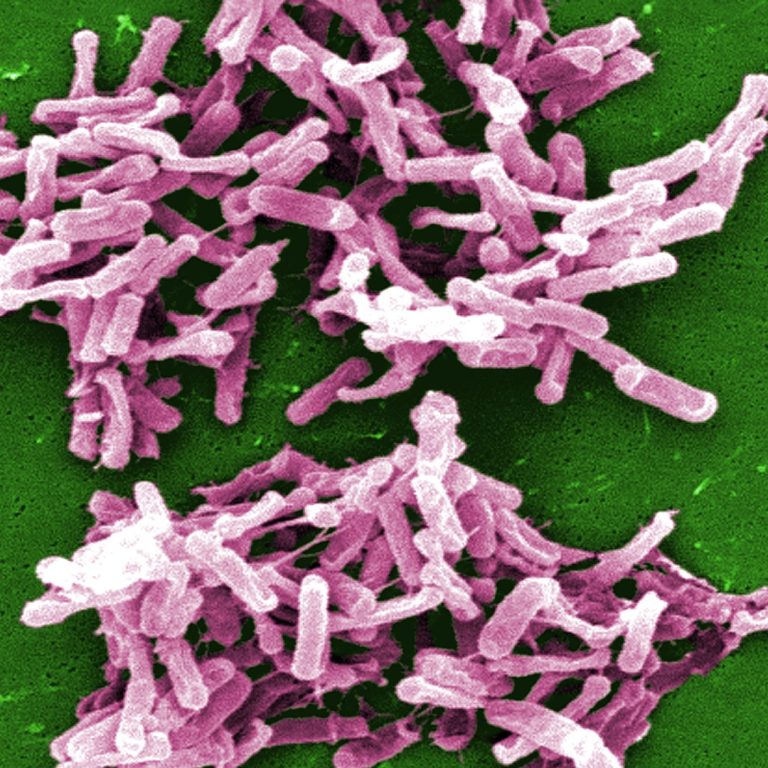
On Apr. 4, 2025, a team of researchers led the University of Utah Health reported that by tracking…

On Apr. 4, 2025, the World Health Organization (WHO) announced that over the past two days, it convened…

On April 3, France’s Université Paris-Saclay said it is one of dozens of European institutions ramping up efforts…

On Apr. 3, 2025, a team led by researchers at Baylor College of Medicine and the Jan and…

On Apr. 3, 2025, a Stanford-led study has found that the gene CXCL12 is connected to the posterior…

On Apr. 3, 2025, The International Journal of Neonatal Screening, an international, peer-reviewed journal focused on newborn screening…

On Apr. 3, 2025, a collaborative research led by investigators at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center…