Labcorp received EUA for at home collection kit for combined COVID-19 and flu detection
On Oct. 1, 2021, LabCorp announced that it had received Emergency Use Authorization (EUA) from the U.S. Food…
On Oct. 1, 2021, LabCorp announced that it had received Emergency Use Authorization (EUA) from the U.S. Food…

On Oct. 1, 2021, Humanigen announced it had submitted all the planned modules as well as a risk…

On Oct. 1, 2021, Merck and Ridgeback Biotherapeutics announced that molnupiravir (MK-4482, EIDD-2801), an investigational oral antiviral medicine,…
On Sept. 30, 2021, the WHO announced that fifteen African countries – nearly a third of the continent’s…

On Sept. 30, 2021, RELIEF THERAPEUTICS reported that the parent company of its U.S. collaboration partner, NRx Pharmaceuticals,…

On Sept. 30, 2021, Trevena announced data from 30 patients enrolled in the proof-of-concept study of TRV027, the…

On Sept. 30 2021, Siemens Healthineers announced the Food and Drug Administration (FDA) 510 (k) clearance of the…
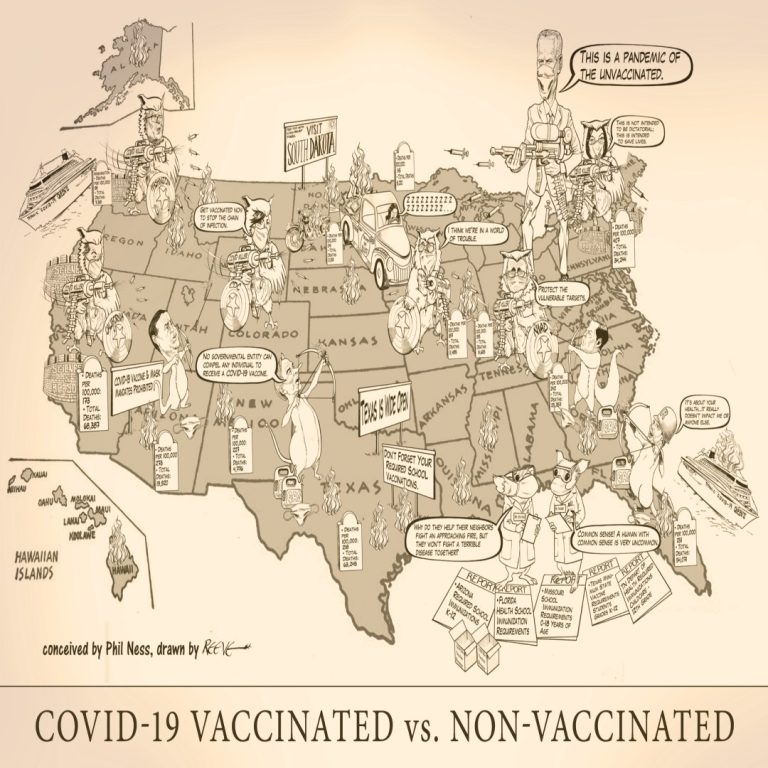
This cartoon illustrates the various issues facing individuals across the U.S. who are vaccinated against COVID-19 verus those…

On Sept. 29, 2021, Colossal, a de-extinction company that is combining the science of genetics with the business…

On Sept. 29, 2021, Pfizer and BioNTech announced they had submitted data to the U.S. Food and Drug…

On Sept. 29, 2021, VBI Vaccines announced positive results from multiple preclinical studies against several COVID-19 variants of…

On Sept. 29, 2021, Cepheid announced it had received Emergency Use Authorization from the U.S. Food & Drug…

On Sept. 29, 2021, the University of Pennsylvania announced that the Breakthrough Prize in Life Sciences was awarded…

On Sept. 29, 2021, Roche confirmed positive data from the phase II/III 2066 study, investigating Ronapreveル (casirivimab and…
On Sept. 29, 2021, Regeneron announced that the New England Journal of Medicine (NEJM) published positive detailed results…
On Sept. 28, 2021, the National Institute of Allergy and Infectious Diseases announced it had awarded approximately $36.3…

On Sept. 28, 2021, the University of Missouri researcher Kamlendra Singh led a team that analyzed protein sequences…

On Sept. 28, 2021, AIM ImmunoTech announced that is had submitted a Pre-Investigational New Drug application (Pre-IND) to…

On Sept. 28, 2021, Sanofi announced that it had terminated plans for its own mRNA-based COVID-19 vaccine given…

On Sept. 28, 2021, Quest Diagnostics reported that one in two (50.5%) American children under six years of…
On Sept. 28, 2021, Pfizer and BioNTech announced they had submitted data to the U.S. Food and Drug…

On Sept. 28, 2021, Soligenix announced publication of pre-clinical immunogenicity studies for CiVax (heat stable COVID-19 vaccine program)…

On Sept. 28, 2021, TGen, an affiliate of City of Hope, will use its MindCrowd website portal to…

On Sept. 27, 2021, Pfizer announced that the first participants have been dosed in a Phase 1 clinical…

On Sept. 27, 2021, RELIEF THERAPEUTICS reported its U.S. collaboration partner, NRx Pharmaceuticals, had announced top line data…

On Sept. 27, 2021, Pfizer announced the start of the Phase 2/3 EPIC-PEP (Evaluation of Protease Inhibition for…

On Sept. 24, 2021, the U.S. Department of Agriculture’s (USDA) announced confirmation of SARS-CoV-2 in a ferret in…

On Sept. 24, 2021, Moderna announced a supply agreement with the government of Peru for 20 million doses…

On Sept. 23, 2021, Twist Bioscience reported that its internally-discovered antibody candidate TB202-3 (CoVIC-094), demonstrated potent binding to…
On Sept. 23, 2021, OraSure Technologies and the Biomedical Advanced Research Development Authority (BARDA) announced up to $13.6…