EUROIMMUN anti-SARS-CoV-2 S1 curve ELISA received FDA Emergency Use Authorization
On Oct. 5, 2021, PerkinElmer announced that the U.S. Food and Drug Administration had provided Emergency Use Authorization…

On Oct. 5, 2021, PerkinElmer announced that the U.S. Food and Drug Administration had provided Emergency Use Authorization…

On Oct. 5, 2021, Johnson & Johnson announced it had submitted data to the U.S. Food and Drug…
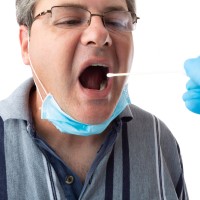
On Oct. 5, 2021, a new report from the U.S. Department of Health and Human Services (HHS) showed…

On Oct. 4, 2021, the Fred & Pamela Buffett Cancer Center announced it had successfully renewed its National…

On Oct. 4, 2021, the Nobel Prize in Physiology or Medicine was awarded to David Julius and Ardem…

On Oct. 4, 2021, the the Secretary of Defense directed the mandatory vaccination of Service members against the…

On Oct. 4, 2021, Pfizer and BioNTech announced that the Committee for Medicinal Products for Human Use (CHMP)…

On Oct. 4, 2021, Dynavax Technologies and the U.S. Department of Defense (DOD) announced Dynavax had executed an…

World’s oldest evidence dating to 23000 BCE for the earliest small-scale plant cultivation was discovered by Dani Nadel…

On Oct. 4, 2021, ACON Laboratories announced that its Flowflexル COVID-19 Antigen Home Test had been authorized for…

Around 8000 BCE, farmers in China, Egypt and Sumeria began using fermentation to produce cheese and wine. One…

A tomb illustration in Memphis, Egypt, depictded a patient being bled from the foot and neck. Though the…

The earliest physical trace of beer dates back to the late fourth millennium BC in present day Iran…

Earliest known evidence of cheese making was found in Croatia dated to 7200 BCE. Residues from sherds of…

Archeological evidence points to human use of pigeons as a food source as early as 10,000 years ago…
The Torah offered instructions for isolating lepers, the first published guidance for health confinement occurred around 400-600 BCE.

In the 16th century BCE the Phoenicians started disseminating the olive throughout the Greek isles, later introducing it…

The Babylonian Code of Hammurabi (ca. 1754 BCE), one of the oldest legal texts in the world, prescribes…
In 583 BCE, the Council of Lyons in Lyon, France restricted lepers from freely associating with healthy persons.

Around 600 BCE, Chinese healers created the first antibiotic – moldy soybean curds – to treat boils.

Around 100 BCE, Chinese growers used powdered chrysanthemum (pyrethrins) as the first insecticide.

Around 500 BCE, Hippocrates, a pioneering physician in the history of Medicine, and considered as the principal author…

On Oct. 2, 2021, the Auburn University College of Veterinary Medicine was announced as the home to the…

On Oct. 1, 2021, Kite, a Gilead Company, announced the U.S. Food and Drug Administration had granted approval…

On Oct. 1, 2021, Humanigen announced it had submitted all the planned modules as well as a risk…
On Oct. 1, 2021, LabCorp announced that it had received Emergency Use Authorization (EUA) from the U.S. Food…

On Oct. 1, 2021, Merck and Ridgeback Biotherapeutics announced that molnupiravir (MK-4482, EIDD-2801), an investigational oral antiviral medicine,…

On Sept. 30, 2021, the WHO announced that fifteen African countries – nearly a third of the continent’s…

On Sept. 30, 2021, RELIEF THERAPEUTICS reported that the parent company of its U.S. collaboration partner, NRx Pharmaceuticals,…

On Sept. 30 2021, Siemens Healthineers announced the Food and Drug Administration (FDA) 510 (k) clearance of the…