U.S. FDA authorizd InteliSwabル COVID-19 rapid tests for at-home testing for delta variant detection
On Nov. 2, 2021, OraSure Technologies announced that the EUA for its InteliSwabル COVID-19 rapid tests had been…
On Nov. 2, 2021, OraSure Technologies announced that the EUA for its InteliSwabル COVID-19 rapid tests had been…

On Nov. 1, 2021, the U.S. Food and Drug Administration (FDA) cleared the first 510(k) for a COVID-19…

On Nov. 1, 2021, Novavax and Serum Institute of India announced that the National Agency of Drug and…

On Nov. 1, 2021, Novavax announced the completion of its rolling submission to Health Canada for authorization of…

On Nov. 1, 2021, for the first time in the U.S., the transmission of COVID-19 from pet parent…

On Oct. 31, 2021, Moderna announced that the U.S. Food and Drug Administration (FDA) had notified the Company…

On Oct. 30, 2021, Penn Medicine officially opened the doors of its 1.5 million-square-foot future-ready Pavilion as clinical…
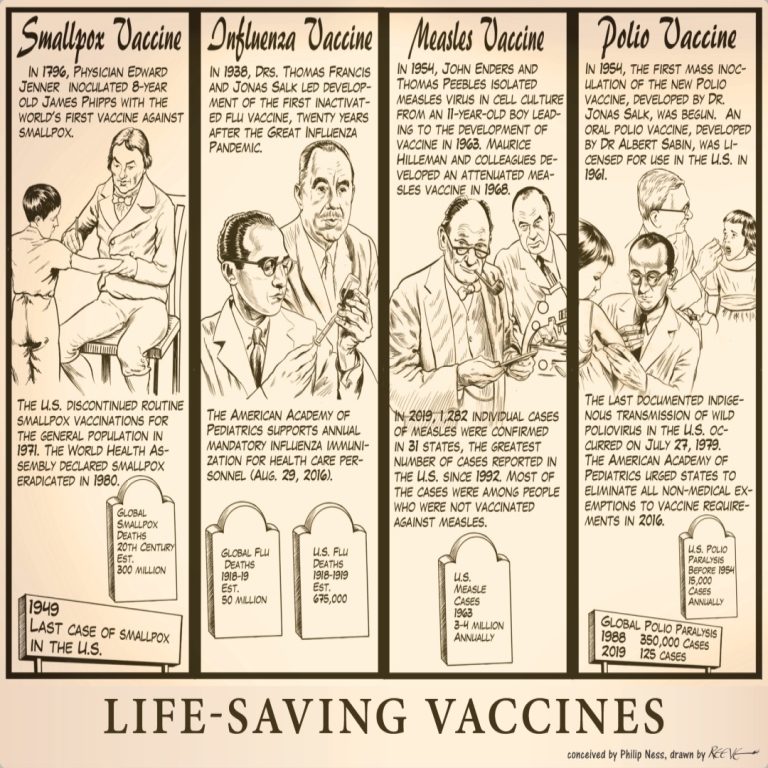
Our Life-Saving Vaccines cartoon illustrates four invaluable vaccines that have affected human civilization throughout history from smallpox and…
On Oct. 29, 2021, Pfizer and BioNTech announced that the U.S. Food and Drug Administration (FDA) had authorized…
On Oct. 29, 2021, research led by scientists at the National Institutes of Health announced they had identified…

On Oct. 29, 2021, Novavax announced the completion of its rolling submission to the Therapeutic Goods Administration (TGA)…
On Oct. 29, 2021, the Centers for Disease Control and Prevention (CDC) published “new science” reinforcing that vaccination…

On Oct. 29, 2021, Oregon Health & Science University’s (OHSU) board of directors approved a project to expand…

On Oct. 28, 2021, Pfizer and BioNTech announced that the U.S. government had purchased 50 million more doses…
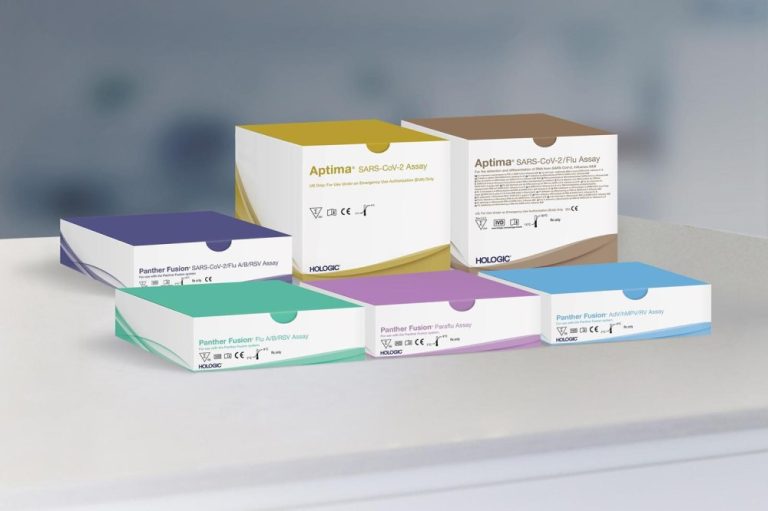
On Oct. 27, 2021, Hologic announced that its Aptima SARS-CoV-2/Flu Assay was available for the simultaneous detection and…

On Oct. 27, 2021, RELIEF THERAPEUTICS announced that its wholly owned subsidiary, APR Applied Pharma Research, reported positive…

On Oct. 27, 2021, Novavax announced the completion of its rolling regulatory submission to the U.K. Medicines and…

On Oct. 27, 2021, Fulgent Genetics announced that it had launched an antibody test for COVID-19 which specifically…

On Oct. 27, 2021, researchers published interim results in The New England Journal of Medicine from a Phase…

On Oct. 27, 2021, the Medicines Patent Pool (MPP) and Merck announced a voluntary licensing agreement to facilitate…

On Oct. 26, 2021, Anixa Biosciences announced that, in conjunction with its partner, Cleveland Clinic, it had commenced…

On Oct. 26, 2021, Vaxart announced that it has dosed the first subject in its Phase II COVID-19…
On Oct. 26, 2021, Pfizer and BioNTech announced that the U.S. Food and Drug Administrationメs (FDA) Vaccines and…

On Oct. 26, 2021, Axcella Therapeutics announced a new clinical program to investigate AXA1125 as a potential treatment…

On Oct. 26, 2021, BioNTech announce that the Company planned to initiate the construction of the first state-of-the-art…

On Oct. 26, 2021, Moderna announced a new Memorandum of Understanding (MoU) to make up to 110 million…

On Oct. 26, 2021, Moderna announced that Swissmedic had authorized a booster dose of Spikevax, the Company’s vaccine…

On Oct. 25, 2021, Merck and Ridgeback Biotherapeutics announced the European Medicines Agency (EMA) had initiated a rolling…

On Oct. 25, 2021, Moderna announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human…

On Oct. 25, 2021, scientists at Oxford University reported that Infection with COVID-19 carried a much higher risk…