Medicago and GSK announced approval by Health Canada of COVIFENZ, an adjuvanted plant-based COVID-19 vaccine
On Feb. 24, 2022, Medicago and GlaxoSmithKline announced that Health Canada had granted approval for COVIFENZ, COVID-19 vaccine,…

On Feb. 24, 2022, Medicago and GlaxoSmithKline announced that Health Canada had granted approval for COVIFENZ, COVID-19 vaccine,…

On Feb. 24, 2022, Novavax shared extended analysis from its pivotal Phase 3 clinical trial conducted in the…

On Feb. 24, 2022, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) confirmed…

On Feb. 24, 2022, BioNTech announced that the Committee for Medicinal Products for Human Use of the European…

On Feb. 24, 2022, Moderna announced that the European Medicines Agency’s Committee for Medicinal Products for Human Use…

On Feb. 24, 2022, researchers from the University of Oxford have used data from UK Biobank participants to…

On Feb. 23, 2022, Moderna and Thermo Fisher Scientific announced a 15-year strategic collaboration agreement to enable dedicated…
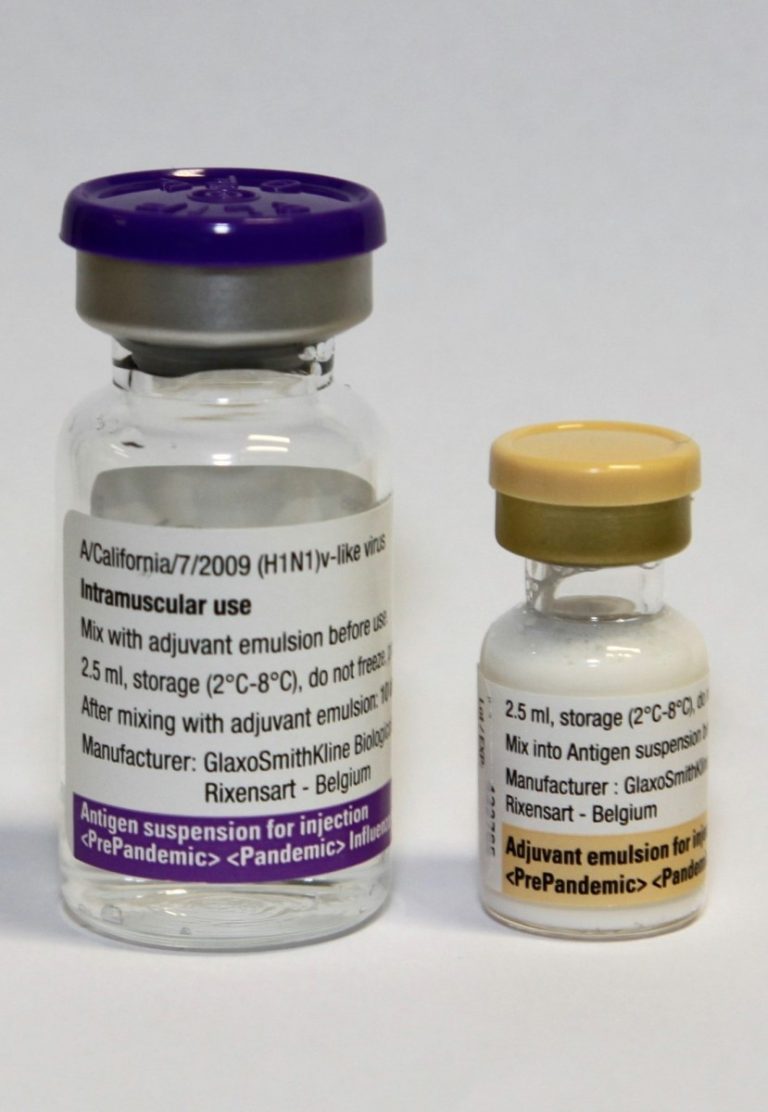
On Feb. 23, 2022, Sanofi and GSK announced that they planned to submit data from both their booster…

On Feb. 23, 2022, Agilent Technologies announced that researchers at the Indian Institute of Technology Bombay in India…

On Feb. 23, 2022, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) confirmed…

On Feb. 23, 2022, Novavax announced the first doses of Nuvaxovid COVID-19 Vaccine (recombinant, adjuvanted) had begun shipping…

On Feb. 22, 2022, Moderna announced a distribution service agreement with Adium Pharma, a leading private Latin American…

On Feb. 22, 2022, Washington University in St. Louis announced that a blood test developed at the University’s…

On Feb. 22, 2022, Sorrento Therapeutics announced that additional preclinical results demonstrate broad spectrum COVISHIELD (STI-9167) neutralizing activity…

On Jan. 24, 2022, researchers from the University of Oxfordメs Big Data Institute announced a major step towards…

On Mar. 22, 2022, Novavax and Serum Institute of India announced that the Drugs Controller General of India…

On Feb. 1, 2022, University of Virginia Cancer Center announced it had been awarded a Comprehensive Cancer Center…

On Feb. 19, 2022, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) confirmed…

On Feb. 18, 2022, the U.S. Centers for Disease Control and Prevention (CDC) released the 2022 recommended immunization…

On Feb. 18, 2022, the WHO announced the first six countries that will receive the technology needed to…

On Feb. 17, 2022, Resverlogix announced that the first Brazilian site had been initiated for its Phase 2b…

On Feb. 17, 2022, Novavax announced that Health Canada had granted authorization for Nuvaxovid COVID-19 Vaccine (Recombinant protein,…

On Feb. 17, 2022, after studying blood samples from 244 patients hospitalized for COVID-19, a group of researchers,…

On Feb. 16, 2022, BioNTech announced it had introduced its approach to establishing scalable vaccine production by developing…

On Feb. 16, 2022, Moderna announced that the Therapeutic Goods Administration in Australia had granted provisional registration for…

On Feb. 16, 2022, the McKelvey School of Engineering at Washington University in St. Louis, announced that it…
On Feb. 15, 2022, a woman with HIV who received a cord blood stem cell transplant to treat…

On Feb. 15, 2022, Pfizer announced that the European Medicines Agency had approved the companyメs 20-valent pneumococcal conjugate…
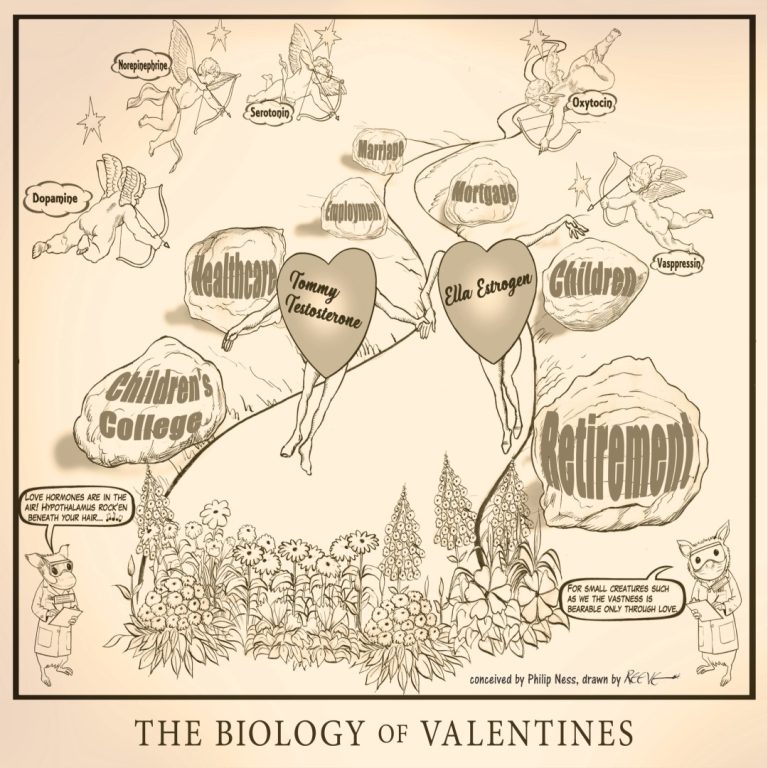
The Biology of Valentines illustrates the biological components of love combined with realities of life. Published Feb. 14,…

On Feb. 14, 2022, the U.S. Department of Agriculture’s (USDA) Animal and Plant Health Inspection Service (APHIS) confirmed…