Washington University’s Jeffrey T. Fort Neuroscience Research building dedicated
On Jan. 25, 2024, dozens of noted scientists, philanthropists, and Washington University, state and local leaders celebrated the…

On Jan. 25, 2024, dozens of noted scientists, philanthropists, and Washington University, state and local leaders celebrated the…
On Jan. 24, 2024, Oregon Health & Science University announced that Pamela Runner, 77, of Corvallis, became the…

On Jan. 22, 2024, the University of California, Davis announced the first reported outbreak of high pathogenicity H5N1…

On Jan. 16, 2024, the U.S. Food and Drug Administration (FDA) announced it had approved CRISPR Therapeutics’ CASGEVY…

On Jan. 15, 2024, CSL announced that Swissmedic had authorised HEMGENIX® (etranacogene dezaparvovec), the first and currently only gene…

On Jan. 12, 2024, the World Health Organization (WHO) announced it had certified Cabo Verde as a malaria-free…

On Jan. 10, 2024, an National Institutes of Health (NIH) supported study announced an n atlas revealing the…

On Jan. 9, 2024, biologists in Alaska have confirmed the first case of a polar bear dying after…

On Jan. 9, 2024, Biogen and Eisai announced that humanized anti- soluble aggregated amyloid-beta (Aβ) monoclonal antibody ‘LEQEMBI’…

On Jan. 9, 2024, the Institute for Health Metrics and Evaluation (HHMI) reported that despite a gradual decline…

On Jan. 5, 2024, the U.S. Food and Drug Administration (FDA) authorized Florida’s Agency for Health Care Administration’s…

On Jan. 4, 2024, France announced it had detected bird flu on a duck farm in the Vendee…

On Jan. 4, 2024, AstraZeneca Sanofi’s Beyfortus (nirsevimab), a long-acting monoclonal antibody, was approved in China for the…

On Dec. 28, 2023, the International Health Regulations National Focal Point (IHR NFP) of Argentina notified the World…
On Dec. 22, 2023, the U.S. Centers for Disease Control and Prevention (CDC) projected the JN.1 variant of…
On Dec. 22, 2023, the U.S. Centers for Disease Control and Prevention (CDC) announced study results that showed…

On Dec. 21, 2023, CSL and and Arcturus Therapeutics announced the results of a Phase 3 study showing…

On Dec. 21, 2023, the World Health Organization (WHO) announced that it had prequalified the R21/Matrix-M malaria vaccine…

On Dec. 20, 2023, the United States Department of Agriculture’s (USDA) National Veterinary Services Laboratory (NVSL) announced it…
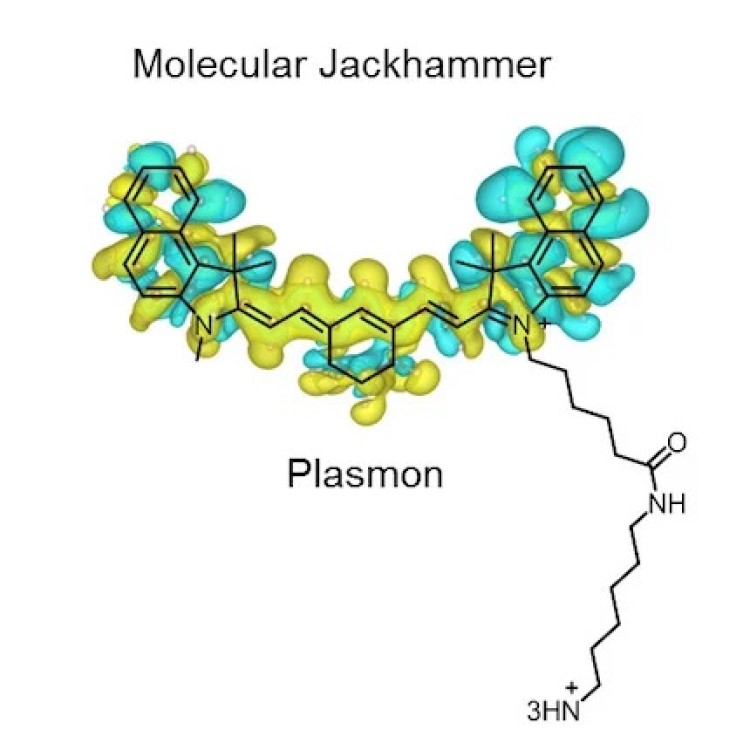
On Dec. 19, 2023, a research team led by Rice University reported they found that the atoms of…

On Dec. 19, 2023, COVAX, the multilateral mechanism for equitable global access to COVID-19 vaccines launched in 2020,…

On Dec. 19, 2023, the U.S. Food and Drug Administration approved the first test that uses DNA in…

On Dec. 18, 2023, the Novo Nordisk Foundation announced it was committing up to DKK 1.8 billion (USD…

On Dec. 18, 2023, W. Kimryn Rathmell, M.D., Ph.D., began work as the 17th director of the National…

On Dec. 18, 2023, the U.S. Centers for Disease Control and Prevention (CDC) announced a study that showed…

On Dec. 18, 2023, Novavax announced that the Taiwan Food and Drug Administration had granted emergency use authorization…

On Dec. 18, 2023, COVID-19 vaccine maker BioNTech (22UAy.DE) announced that it aims to start production at its…

On Dec. 15, 2023, In a pivotal move towards addressing one of the worldメs most underrecognized health challenges,…

On Dec. 14, 2023, the Department of Energy’s (DOE) Pacific Northwest National Laboratory (PNNL) announced that it had…

On Dec. 14, 2023, The Coalition for Epidemic Preparedness Innovations (CEPI) and PATH today announced a renewed collaboration…