The US Dept of Defense launched effort to collect COVID-19 convalescent plasma
On Jun. 1, 2020, U.S. Dept. of Defense announced it had begun an effort to collect 8,000 donated…

On Jun. 1, 2020, U.S. Dept. of Defense announced it had begun an effort to collect 8,000 donated…
On Jun. 1, 2020, the Government of the Democratic Republic of the Congo announced that a new outbreak…
On Jun. 1, 2020, Gilead Sciences announced topline results from the Phase 3 SIMPLE trial in hospitalized patients…

On Jun. 1, 2020, Emergent BioSolutions announced it had been issued a task order under an existing contract…
On Jun. 1, 2020, Anixa Biosciences and OntoChem announced they had synthesized four potential Covid-19 compounds that will…

On Jun. 1, 2020, the USDA announced it is transitioning its virulent Newcastle disease (vND) efforts in southern…

On Jun. 1, 2020, Eli Lilly announced patients had been dosed in the world’s first study of a…

On Jun. 1, 2020, Proteostasis Therapeutics announced results from in vitro studies evaluating the use of PTI-129 as…

On Jun. 1, 2020, the FDA issued an emergency use authorization (EUA) for Impella RP to include patients…

On Jun. 1, 2020, Altimmune announced the FDA had authorized the Company to proceed with a clinical trial…

On Jun. 1, 2020, LabCorpᆴ announced that Covance, its drug development business, had created COVID-19 Clinical Trial Connect…

On Jun. 1, 2020, OPKO Health announced the FDA had authorized OPKO to undertake a Phase 2 trial…

On Jun. 1, 2020, Tonix Pharmaceuticals announced an agreement whereby FUJIFILM Diosynth Biotechnologies will provide contract manufacturing and…

On Jun. 1, 2020, SIGA Technologies announced its first international delivery of TPOXX (tecovirimat), with 2,500 courses delivered…

On Jun. 1, 2020, PathGroup announced the availability of a new, minimally-invasive nasal swab collection option as a…
On May 29, 2020, the World Health Organization (WHO) announced that thirty countries and multiple international partners and…

On May 29, 2020, the FDA took steps to further support the development of COVID-19 tests for at-home…

On May 29, 2020, the Oxford Foundry announced support of four new innovative solutions for the OXFO COVID-19…

On May 29, 2020, the California Institute for Regenerative Medicine (CIRM) approved investments in three early-stage research programs…

On May 29, 2020, Moderna announced the first participants in each age cohort had been dosed in the…
On May 29, 2020, Johnson & Johnson announced that its Janssen Pharmaceutical subsidiary received a positive opinion from…

On May 28, 2020, a team of researchers has generated a developmental map of a key sound-sensing structure…

On May 28, 2020, the Jenner Institute, University of Oxford, UK and Advent Srl ヨ an IRBM company,…
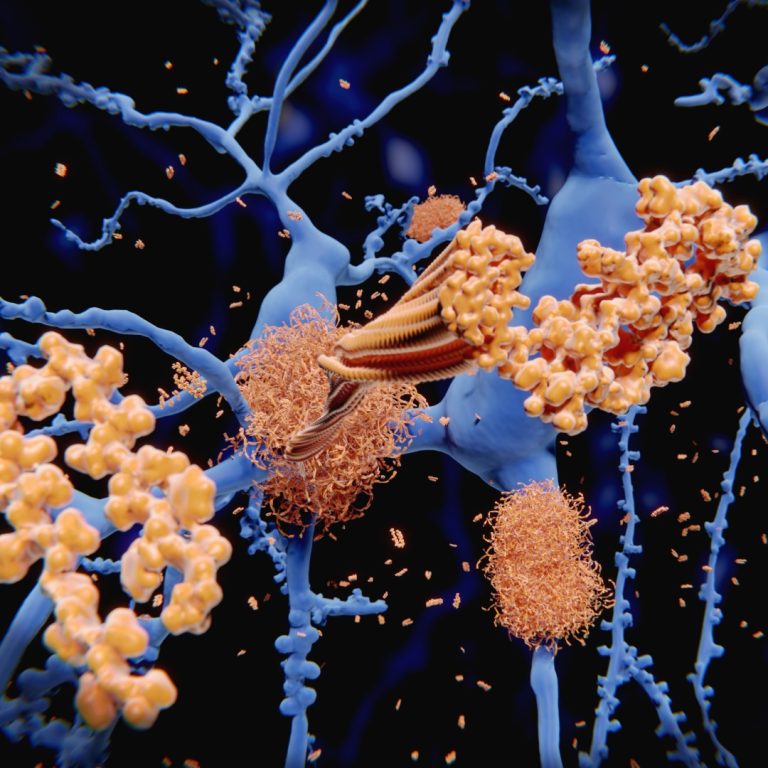
On May 28, 2020, the FDA approved Avid Radiopharmaceuticals’ Tauvid (flortaucipir F18) for intravenous injection, the first drug…

On May 28, 2020, GSK confirmed its intention to manufacture 1 billion doses of its pandemic vaccine adjuvant…
On May 28, 2020, the U.S. Dept. of Veterans Affairs (VA) began a national four-year study on the…

On May 28, 2020, Biogen and the Lemelson-MIT Program (LMIT) at the Massachusetts Institute of Technology announced the…

On May 28, 2020, Vaxxas announced that Merck had exercised its option to utilize Vaxxasメ proprietary High Density…

On May 28, 2020, Quest Diagnostics announced it had received emergency use authorization (EUA) from the FDA for…

On May 28, 2020, Roche announced it had initiated of a global phase III, randomised, double-blind, multicentre study…