Todos Medical entered Into COVID-19 PCR testing implementation and equipment financing partnership with AID Genomics
On Sept. 25, 2020, Todos Medical announced that it had entered into an implementation and equipment financing partnership…

On Sept. 25, 2020, Todos Medical announced that it had entered into an implementation and equipment financing partnership…

On Sept. 24, 2020, Lumen Bioscience and the US Army Medical Research and Development Command, announced an agreement…

On Sept. 24, 2020, an international research team led by the Max Planck Institute for Evolutionary Anthropology in…

On Sept. 24, 2020, new findings reported by scientists at the National Institutes of Health and their collaborators…

On Sept. 24, 2020, a team of scientists announced they had created the first atlas of human heart…

On Sept. 24, 2020, the U.S. Department of Health and Human Services and the U.S. Food and Drug…

On Sept. 24, 2020, Yale University announced it had designated three independent laboratories to perform the university-developed SalivaDirect…

On Sept. 24, 2020, Novavax announced that it had initiated its first Phase 3 study to evaluate the…

On Sept. 23, 2020, a fourth Phase 3 clinical trial evaluating an investigational vaccine for coronavirus disease 2019…
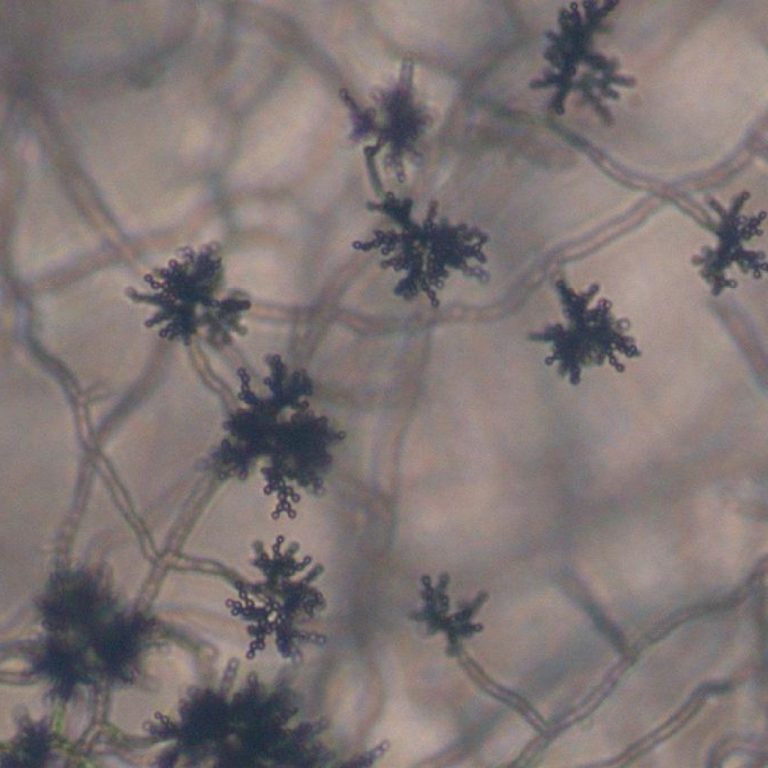
On Sept. 23, 2020, the USDA Agricultural Research Service (ARS) announced that a harmless airborne fungus, Cladosporium sphaerospermum…

On Sept. 23, 2020, the WHO with the UN, specialised agencies and partners called on countries to develop…

On Sept. 23, 2020, Sinovac Biotech announced that the inactivated COVID-19 vaccine candidate developed by Sinovac Life Sciences,…

On Sept. 23, 2020, the FDA issued an emergency use authorization (EUA) for the first serology (antibody) point-of-care…

On Sept. 23, 2020, the CDC announced the extension of a No Sail Order for cruise ships through…

On Sept. 23, 2020, the CDC awarded $200 million to 64 jurisdictions through the existing Immunizations and Vaccines…

On Sept. 23, 2020, Humanigen announced a strategic collaboration with Thermo Fisher Scientific to expand the manufacturing capacity…

On Sept. 23, 2020, Johnson & Johnson announced the launch of its large-scale, pivotal, multi-country Phase 3 trial…
On Sept. 23, 2020, Pascal Biosciences announced the Company had confirmed an earlier report that certain cannabinoids block…

On Sept. 22, 2020, the NIH annunced that two randomized, placebo-controlled clinical trials expanded enrollment to further evaluate…

On Sept. 22, 2020, the FDA announced it had launched the Digital Health Center of Excellence within the…

On Sept. 22, 2020, RedHill Biopharma announced approval from the Brazilian Health Regulatory Agency (ANVISA) for its ongoing…
On Sept. 22, 2020, Sanofi and GSK announced they had signed agreements with the Government of Canada for…

On Sept. 22, 2020, Sinovac Biotech announced that it had recently commenced phase III clinical trials for its…
On Sept. 22, 2020, Oxford Immunotec announced that it had entered into an extensive research collaboration with Public…

On Sept. 22, 2020, Codagenix announced that the Serum Institute of India has begun manufacturing CDX-005, the company’s…

On Sept. 22, 2020, BioSig Technologies and its subsidiary, ViralClear Pharma announced that the size of the ongoing…

On Sept. 21, 2020, a study comparing the immune responses of adults and children with COVID-19 detected key…

On Sept. 21, 2020, XBiotech announced it was developing a new breakthrough candidate therapy it calls FLUVID for…

On Sept. 18, 2020, Roche announced the launch of its Elecsysᆴ Anti-SARS-CoV-2 S antibody test for markets accepting…

On Sept. 18, 2020, Sanofi and GSK finalised and signed an advanced purchase agreement with the European Commission…